Muddiest Point- Phase Diagrams II: Eutectic Microstructures
TLDRThis screencast delves into the microscopic structures associated with phase diagrams, focusing on how they vary with composition and affect material strength. It explains the significance of the eutectic composition for applications like soldering due to its optimal strength and lowest melting point. The video guides viewers through understanding single-phase and two-phase region microstructures, illustrating the formation of alternating platelets in the eutectic region and the impact on strength. It also covers hypo- and hyper-eutectic microstructures, clarifying how phase weight fractions and chemical compositions relate to the observed microstructures. The screencast concludes by summarizing the five microstructures found at 182°C and elucidating why the eutectic microstructure offers the highest strength due to barriers to dislocation motion.
Takeaways
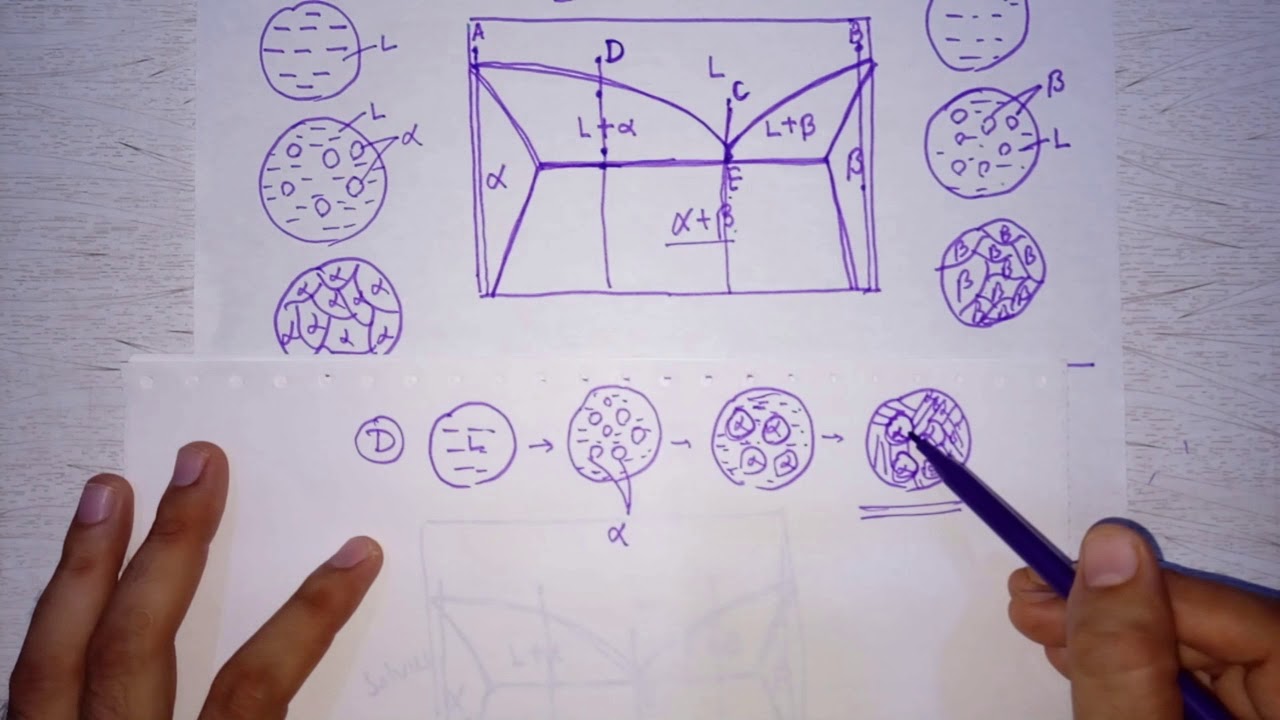
- 🔍 The video discusses microstructures associated with phase diagrams, focusing on how they appear and their relation to phase fractions.
- 📚 It explains that microstructures vary with composition and significantly affect the material's strength, with the highest strength occurring at the eutectic (TIC) composition.
- 🔨 The eutectic composition is particularly useful in applications like soldering due to its lowest melting point and highest strength.
- 🌡️ The tutorial covers different scenarios, such as single-phase regions and two-phase regions, explaining the microstructures at various temperatures and compositions.
- 🧪 In single-phase regions, the material is entirely liquid or solid with a uniform composition and phase weight fraction of 1.0.
- 📉 The size of the area in a microstructure diagram corresponds to the phase fraction, indicating the proportion of each phase present.
- 🛠️ At the eutectic composition, the microstructure consists of alternating platelets of alpha and beta, which form due to the geometry providing a short path for atoms to solidify.
- 🔬 Hypoeutectic and hypereutectic microstructures are explained, showing how cooling affects the phase composition and the resulting microstructure.
- 💪 The eutectic microstructure has the highest strength because it has the most barriers to dislocation motion, making it difficult for the material to deform.
- 📊 The video concludes by summarizing the five microstructures found at 182°C for different compositions, highlighting the relationship between composition and microstructure.
- 🤔 The script invites viewers to ask questions in the comment section, emphasizing the interactive and educational nature of the content.
Q & A
What is the main focus of the second part of the phase diagrams series in the screencast?
-The second part of the series focuses on the microstructures associated with phase diagrams, explaining how to determine what microstructures look like, how the size of the area in the microstructure diagram relates to phase fraction, and the conditions under which different microstructures form.
Why are microstructures important in the context of phase diagrams?
-Microstructures are important because they vary with composition and have a direct effect on the strength of materials. Understanding microstructures can help in applications such as soldering, where different compositions have different strengths and melting points.
What is the significance of the TIC composition in terms of strength and melting point?
-The TIC (eutectic) composition is significant because it produces the highest strength and the lowest melting point. This makes it ideal for applications like soldering for microchips, where the solder with the TIC composition is commonly used.
What does the microstructure look like in a single-phase region with a temperature of 325 and an overall composition of 10% tin?
-In a single-phase region with these conditions, the microstructure consists of a liquid phase with a chemical composition of 10% tin and a phase weight fraction of 1.0, as there is only one phase present.
How does the microstructure of single-phase alpha differ from single-phase liquid?
-Single-phase alpha is a solid phase consisting of polycrystalline alpha with no beta or liquid present. It is characterized by grains of the alpha phase, whereas single-phase liquid does not have a microstructure as it is just liquid.
What happens to the microstructure when the temperature is 1° below the TIC temperature?
-At 1° below the TIC temperature, the microstructure consists of single-phase alpha, with a chemical composition of 10% tin and a phase weight fraction of 1.0, as the system is in the solid phase region.
What is the relationship between the phase weight fraction and the size of the phase in the microstructure diagram?
-The phase weight fraction is approximately equal to the amount of area that the phase takes up in the microstructure diagram. This relationship helps in visualizing the proportion of each phase in the microstructure.
Can you explain the formation of alternating platelets of alpha and beta in the TIC composition?
-When the liquid cools down to the TIC composition, it solidifies into alternating platelets of alpha and beta. This occurs because the geometry of adjacent platelets provides a short path for atoms to solidify, with alpha rejecting tin and beta rejecting lead.
What are hypoeutectic and hypereutectic microstructures, and how do they differ?
-Hypoeutectic microstructures form when the overall composition is below the eutectic point, resulting in crystalline chunks of alpha solidifying out of the liquid. Hypereutectic microstructures form when the overall composition is above the eutectic point, resulting in crystalline chunks of beta forming as the liquid cools.
Why does the TIC microstructure have the highest strength among the different microstructures?
-The TIC microstructure has the highest strength because it has the most barriers to dislocation motion. The alternating platelets of alpha and beta block dislocation motion effectively, making the material stronger compared to single-phase regions or regions with primary chunks.
Outlines
📚 Introduction to Microstructures in Phase Diagrams
This paragraph introduces the second part of a series on phase diagrams, focusing on the microstructures associated with them. It outlines the questions to be answered in the screencast, such as determining the appearance of microstructures, understanding the relationship between the size of areas in the microstructure diagram and phase fraction, and explaining the conditions for alternating platelets versus blobs of alpha or beta. The paragraph also delves into why maximum strength occurs at the eutectic (TIC) composition, using soldering as an example to illustrate the practical applications of these properties. The screencast aims to educate viewers on single-phase region microstructures, starting with a temperature of 325 degrees and an overall composition of 10% tin, which places the scenario in the single-phase liquid region with a uniform chemical composition and phase weight fraction.
🔍 Exploring Single-Phase and Two-Phase Microstructures
The second paragraph delves deeper into the specifics of single-phase microstructures, illustrating what they look like at different temperatures and compositions. It explains the process of determining the microstructure for a given set of conditions, such as a temperature one degree below the eutectic temperature with 10% tin composition, resulting in a single-phase alpha structure. The paragraph also covers how to calculate the chemical composition and phase weight fraction for different regions of the phase diagram. It transitions into discussing two-phase microstructures, such as the formation of alternating platelets of alpha and beta at the eutectic composition, and the significance of phase weight fraction in relation to the area occupied by each phase in the diagram.
🌡️ Cooling Effects on Hypoeutectic and Hypereutectic Structures
This paragraph examines the effects of cooling on hypoeutectic and hypereutectic microstructures. It starts by describing the microstructure at a temperature of 300°C and 40% tin, which places the scenario in the liquid region. As the temperature is lowered to 225°C, the microstructure evolves into crystalline chunks of alpha solidifying out of the liquid, with the liquid's tin composition increasing due to the rejection of tin by alpha. The paragraph further explores the changes in microstructure and composition as the temperature is lowered to one degree above the eutectic temperature, resulting in a two-phase region of alpha and liquid with distinct chemical compositions and phase weight fractions.
🛠️ Formation of Primary Beta and Alternating Platelets
The fourth paragraph discusses the formation of primary beta and the resulting microstructures when cooling a liquid with an overall composition of 85% tin. It explains the process of crystallization, where crystalline chunks of beta form by taking in tin from the liquid, causing the liquid's composition to decrease. As the temperature is further reduced to 184°C, the microstructure shows an increase in the phase weight fraction of beta, with the liquid's composition approaching the eutectic composition. The paragraph concludes with the scenario of cooling to one degree below the eutectic temperature, resulting in a beta plus alpha region with distinct compositions and phase weight fractions for both phases.
🏗️ Summary of Microstructures and Their Strength Implications
In the final paragraph, the screencast summarizes the five microstructures found at 182°C across different compositions. It describes the microstructures for compositions ranging from 10% to 99% tin, highlighting the transition from single-phase polycrystalline alpha to crystalline alpha with eutectic alternating platelets, to just alternating platelets at the eutectic composition, and finally to crystalline chunks of protic beta with eutectic platelets, and single-phase polycrystalline beta. The paragraph concludes by explaining the strength implications of these microstructures, particularly why the eutectic microstructure has the highest strength due to the presence of alternating platelets that act as barriers to dislocation motion.
Mindmap
Keywords
💡Phase Diagrams
💡Microstructure
💡Strength
💡Eutectic Composition (TIC Composition)
💡Single-Phase Region
💡Phase Weight Fraction
💡Polycrystalline
💡Alternating Platelets
💡Hypoeutectic and Hypereutectic Structures
💡Dislocation Motion
Highlights
The screencast discusses microstructures associated with phase diagrams, focusing on their appearance and relationship to phase fractions.
The highest strength occurs at the eutectic (TIC) composition, which also has the lowest melting point, making it ideal for soldering applications.
In the single-phase region, the microstructure is a homogeneous liquid or solid phase with a phase weight fraction of 1.0.
Microstructures in the two-phase region consist of alternating platelets of alpha and beta phases, formed as the liquid solidifies.
The phase weight fraction is approximately equal to the area that the phase occupies in the diagram.
Hypoeutectic microstructures involve crystalline chunks of alpha solidifying out of the liquid, increasing the liquid's tin content.
Cooling a hypoeutectic liquid further results in an increase in alpha phase size and a higher phase weight fraction.
Hypereutectic microstructures begin with liquid cooling and the formation of crystalline chunks of beta by rejecting lead.
Further cooling of hypereutectic compositions leads to an increase in beta phase size and a change in the liquid's composition towards the eutectic.
At the eutectic composition, the microstructure consists of alternating platelets of eutectic alpha and beta, maximizing strength.
Primary alpha and beta chunks that form above the eutectic temperature are distinguishable from those formed at the eutectic temperature.
The eutectic microstructure has the highest strength due to the presence of numerous barriers to dislocation motion.
Single-phase regions lack the alternating platelets, resulting in weaker structures and less effective blocking of dislocation motion.
The screencast concludes by summarizing the five microstructures found at 182°C with varying compositions.
Understanding microstructures is crucial for applications where material strength and properties are critical.
The screencast provides a comprehensive guide to interpreting phase diagrams and predicting microstructures.
Engineering applications, such as soldering for microchips, rely on the principles explained in the screencast for material selection.
Transcripts
Browse More Related Video
Lecture 17 Microstructures on eutectic and eutectoid phase diagram

Muddiest Point- Phase Diagrams I: Eutectic Calculations and Lever Rule

FE Exam Review - FE Mechanical - Material Properties - Phase Diagrams

Eutectic reaction

Introduction to Materials Engineering: CH9

Microstructure of a Hypoeutectoid Steel
5.0 / 5 (0 votes)
Thanks for rating: