Microstructure of a Hypoeutectoid Steel
TLDRThis script delves into the microstructure of hypoeutectoid steel, which contains less than 0.8% carbon. It explains the phase transformations from a single-phase Austenite to a two-phase region of Gamma plus Alpha, leading to the formation of proeutectoid Ferrite at grain boundaries. As the steel cools, more Alpha phase forms, and at 725°C, the remaining Austenite transforms into Pearlite through the eutectoid reaction. The final microstructure consists of proeutectoid Ferrite and Pearlite, with the proportions varying based on the steel's carbon content. The script also distinguishes between eutectoid and eutectic reactions, emphasizing the importance of understanding phase diagrams for material properties.
Takeaways
- 📚 The script discusses the microstructure of hypoeutectoid steel, which has a carbon content less than 0.8%.
- 🔍 Hypoeutectoid steel is characterized by phases such as Austenite (Gamma), Ferrite (Alpha), and Cementite (Fe3C), and exists in a two-phase region of Gamma plus Alpha.
- 🌡️ At point A in the script, the microstructure is single-phase Austenite, which is polycrystalline with visible grain boundaries.
- 📉 Crossing the Gamma plus Alpha phase boundary introduces the formation of Alpha phase, starting at grain boundaries due to heterogeneous nucleation.
- 🔄 As the steel cools, the Lever Rule dictates the increasing fraction of Alpha phase relative to the remaining Austenite.
- 🔥 At 725 degrees Celsius, the remaining Austenite of 0.8% carbon undergoes the eutectoid reaction, transforming into Alpha plus Fe3C, known as Pearlite.
- 📍 The hypoeutectoid steel's microstructure at point C consists of proeutectoid Ferrite (formed before the eutectoid reaction) and Pearlite.
- 🔑 The term 'proeutectoid' is used to distinguish Alpha phase that formed prior to the eutectoid reaction from that formed during it.
- 📈 The amount of proeutectoid Ferrite can be calculated using the Lever Rule on a tie line just above the eutectoid horizontal.
- 🔄 The fraction of proeutectoid Alpha decreases as the carbon content (C naught) increases, with the amount of Pearlite increasing correspondingly.
- 🧠 The script emphasizes the importance of understanding the differences between eutectoid and eutectic reactions, both involving the decomposition of a single phase into two solid phases but initiated from different states (solid vs. liquid).
Q & A
What is the definition of hypoeutectoid steel?
-Hypoeutectoid steel is defined as a steel with a carbon content less than the eutectoid composition of 0.8%.
What are the phases present in the microstructure of hypoeutectoid steel?
-The phases in the microstructure of hypoeutectoid steel include Austenite (Gamma phase), Ferrite (Alpha phase), and Fe3C (Cementite).
What is the significance of the term 'proeutectoid' in the context of hypoeutectoid steel?
-Proeutectoid refers to the phase that forms before the eutectoid reaction. In hypoeutectoid steel, proeutectoid Ferrite (Alpha phase) forms at the grain boundaries during cooling.
How does the microstructure at point A in the script differ from point B?
-At point A, the microstructure is a single phase Austenite, while at point B, it is a two-phase region consisting of both Austenite and Ferrite (Alpha phase).
What is the role of grain boundaries in the formation of the Alpha phase in hypoeutectoid steel?
-Grain boundaries serve as preferred sites for heterogeneous nucleation, where the new Alpha phase tends to form during the cooling process.
What is the significance of the eutectoid horizontal at 725 degrees Celsius?
-The eutectoid horizontal at 725 degrees Celsius is the temperature and composition at which the remaining Austenite phase transforms into a mixture of Alpha and Fe3C, known as Pearlite, through the eutectoid reaction.
What is the difference between eutectoid and eutectic reactions?
-Eutectoid and eutectic reactions both involve the decomposition of a single phase into two solid phases upon cooling. The difference lies in the nature of the high-temperature phase: in eutectic, it is a liquid phase, while in eutectoid, it is a solid phase (Austenite).
How can the amount of proeutectoid Ferrite be determined in a hypoeutectoid steel?
-The amount of proeutectoid Ferrite can be determined using the Lever Rule on a tie line just above the eutectoid horizontal, which is between the composition of the steel and 0.8% carbon.
What is the final microstructure of hypoeutectoid steel after cooling?
-The final microstructure of hypoeutectoid steel consists of proeutectoid Ferrite and Pearlite, where Pearlite is a mixture of Alpha and Fe3C formed through the eutectoid reaction.
How does the amount of carbon in hypoeutectoid steel affect the fraction of proeutectoid Ferrite and Pearlite?
-As the carbon content (C naught) in hypoeutectoid steel increases towards 0.8%, the fraction of proeutectoid Ferrite decreases, while the fraction of Pearlite increases. At 0.8% carbon, there is no proeutectoid Ferrite, and the steel consists of 100% Pearlite.
What is the purpose of the Lever Rule in the context of phase transformations in steel?
-The Lever Rule is used to determine the fractions of different phases formed during phase transformations, such as the amount of Alpha phase formed at different stages of cooling in hypoeutectoid steel.
Outlines
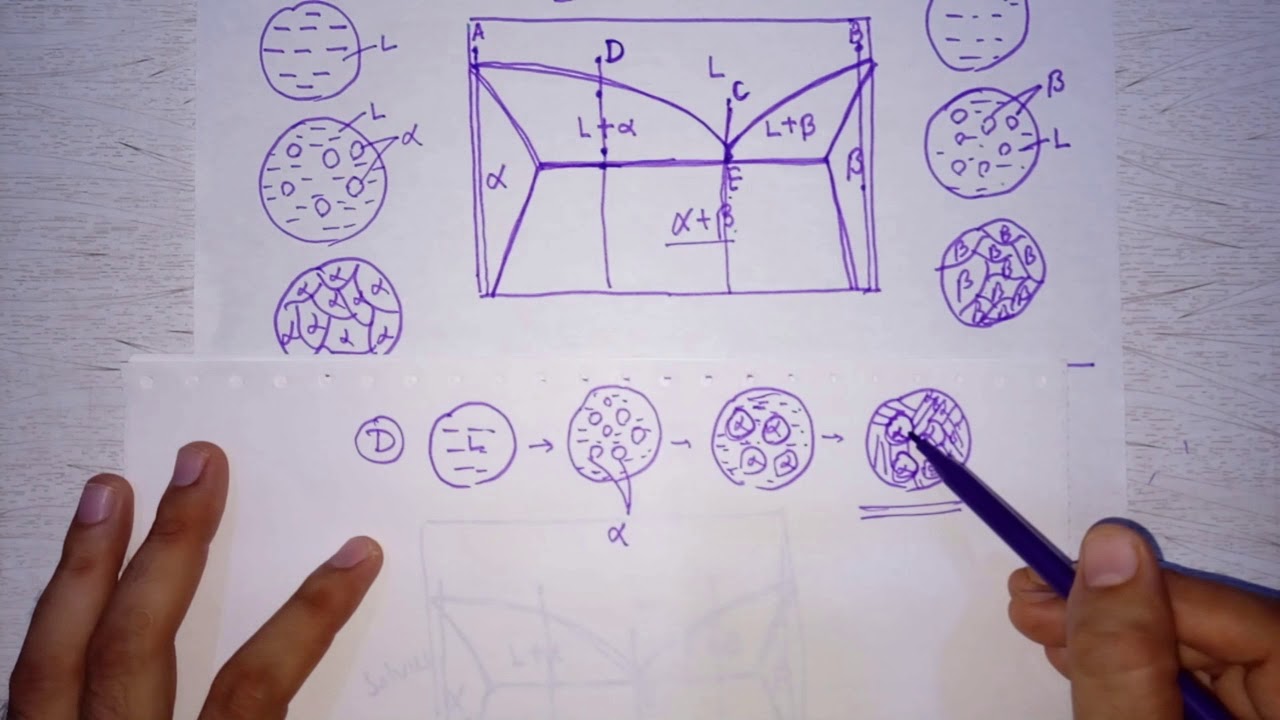
🔍 Microstructure of Hypoeutectoid Steel
This paragraph introduces the microstructure of hypoeutectoid steel, which contains less than 0.8% carbon, contrasting it with eutectoid steel. The speaker explains the phase diagram, highlighting the phases: Austenite (Gamma), Ferrite (Alpha), and Cementite (Fe3C). The microstructure at three different points (A, B, C) on the cooling curve is discussed. At point A, the steel is single-phase Austenite, while at B, it enters the two-phase region of Gamma plus Alpha, where Alpha phase starts forming at grain boundaries due to heterogeneous nucleation. As the steel cools further, more Alpha phase forms until it reaches the eutectoid temperature of 725°C, where the remaining Austenite transforms into Pearlite, a mixture of Alpha and Fe3C.
📉 Phase Transformation at Eutectoid Temperature
The second paragraph delves into the phase transformation that occurs at the eutectoid temperature of 725°C. It explains that at this point, the remaining Austenite, which has a carbon concentration of 0.8%, transforms into Pearlite through the eutectoid reaction. The microstructure at point C is expected to consist of the original Ferrite (Alpha) that formed earlier, and the remaining Austenite that has now transformed into Pearlite. The distinction between proeutectoid Ferrite, which forms before the eutectoid reaction, and the Pearlite that forms during the reaction, is emphasized. The speaker also clarifies the difference between eutectoid and eutectic reactions, noting that the former involves a solid phase decomposing into two solid phases, while the latter involves a liquid phase decomposing into two solid phases.
📏 Calculating Proeutectoid Ferrite and Pearlite Ratios
The final paragraph focuses on calculating the ratios of proeutectoid Ferrite and Pearlite in hypoeutectoid steel. It describes the use of the Lever Rule on a tie line just above the eutectoid horizontal to determine the fraction of proeutectoid Alpha (Ferrite) based on the steel's composition. The formula provided calculates the fraction of proeutectoid Alpha as the difference between 0.8 and the steel's carbon content (C naught), divided by the total carbon range for the phase field (0.8 - 0.02). As C naught increases, the amount of proeutectoid Alpha decreases, and the amount of Pearlite increases, reaching 100% Pearlite at the eutectoid composition of 0.8% carbon.
Mindmap
Keywords
💡Eutectoid Steel
💡Hypoeutectoid Steel
💡Austenite
💡Ferrite
💡Cementite
💡Proeutectoid Ferrite
💡Pearlite
💡Eutectoid Reaction
💡Lever Rule
💡Microstructure
💡Heterogeneous Nucleation
Highlights
Introduction to microstructure of hypoeutectoid steel, which has less than 0.8% carbon content compared to eutectoid steel.
Explanation of phases in the diagram: Austenite (Gamma), Ferrite (Alpha), and Fe3C (Cementite).
Description of the two-phase region of Gamma plus Alpha and Alpha plus Fe3C.
Microstructure at point A consists of a single-phase Austenite, which is polycrystalline Gamma.
Crossing the Gamma plus Alpha boundary leads to the formation of Alpha phase.
Heterogeneous nucleation of Alpha phase often occurs at grain boundaries.
As the steel cools, the fraction of Alpha phase increases according to the Lever Rule.
At 725°C, the remaining Austenite has a composition of 0.8% carbon, ready for the eutectoid reaction.
Further cooling leads to the transformation of remaining Austenite into Pearlite through the eutectoid reaction.
Microstructure at point C consists of proeutectoid Ferrite (Alpha) and Pearlite.
Proeutectoid Ferrite forms before the eutectoid reaction and is distinct from Alpha formed during the reaction.
Pearlite is a mixture of Alpha and Fe3C, formed during the eutectoid reaction.
Hypoeutectoid steel microstructure consists of proeutectoid Ferrite and Pearlite.
Calculation of proeutectoid Ferrite and Pearlite fractions using the Lever Rule and tie lines.
The relationship between eutectic and eutectoid reactions, and the use of proeutectic and proeutectoid terms.
Eutectoid steel, in contrast, transforms entirely into Pearlite at 0.8% carbon content.
The amount of proeutectoid Ferrite decreases as the steel composition approaches 0.8% carbon.
At 0.8% carbon, the hypoeutectoid steel microstructure is 100% Pearlite, similar to eutectoid steel.
Transcripts
Browse More Related Video

Eutectoid, Hypoeutectoid and Hypereutectoid steels

Fe-C phase diagram

Microstructure of a Hypereutectoid Steel (Contd)

Muddiest Point- Phase Diagrams III: Fe-Fe3C Phase Diagram Introduction

Why is the carbon content in steel so important?
Lecture 17 Microstructures on eutectic and eutectoid phase diagram
5.0 / 5 (0 votes)
Thanks for rating: