The Strong Nuclear Force
TLDRThis video introduces the concept of the strong nuclear force, one of the four fundamental forces of nature, which holds protons together in an atom's nucleus despite their electromagnetic repulsion. Discovered in the early 20th century, the strong force is about 100 times stronger than the electromagnetic force but acts over very short distances, similar to Velcro. This explains the stability of lighter nuclei and the instability of heavier ones. The video promises a follow-up on how the strong force operates within particle physics, highlighting its significance as the second strongest known force in the universe.
Takeaways
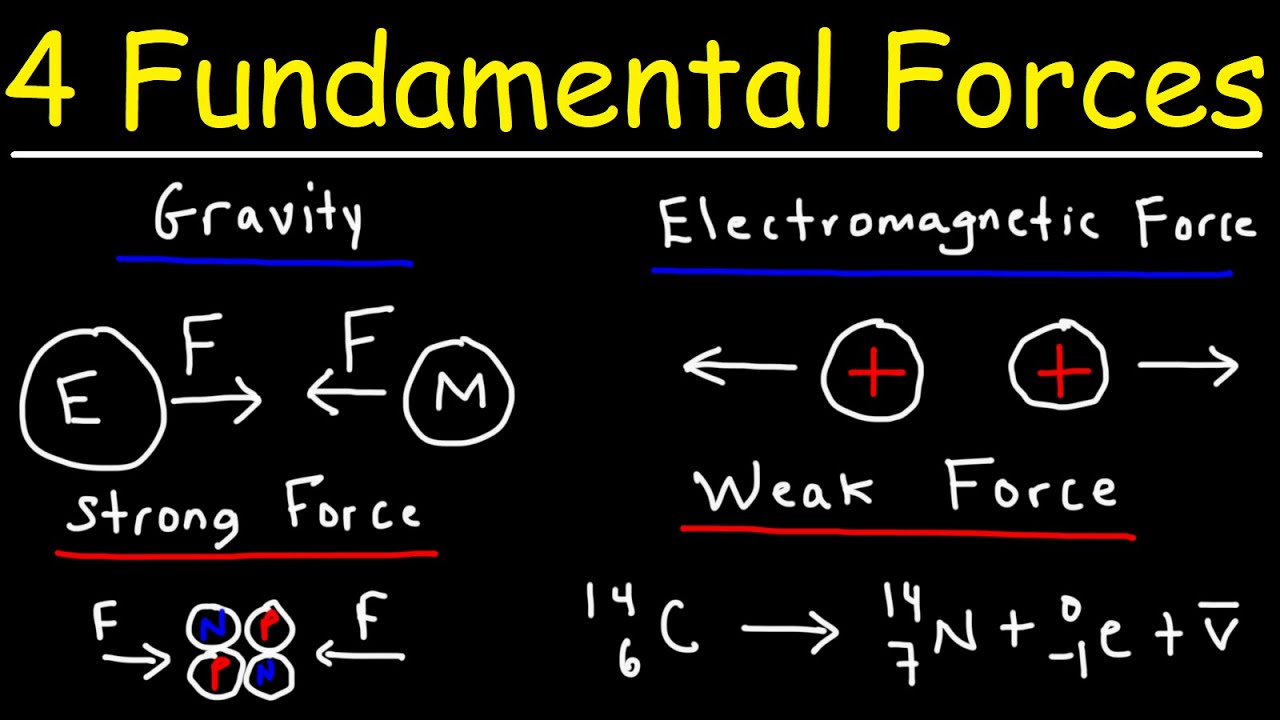
- 🔬 There are four fundamental forces in particle physics: gravity, electromagnetism, the strong nuclear force, and the weak nuclear force.
- 🔍 Ernest Rutherford discovered the basic structure of the atom in 1911, which includes a nucleus and orbiting electrons.
- 🚀 In 1917, Rutherford discovered the proton, which led to the understanding that the nucleus is composed of smaller positively charged particles.
- 💥 The electromagnetic force between protons repels them, with a significant force of about 20 pounds for just two protons.
- 🌌 The strong nuclear force is the force that keeps protons together in the nucleus despite the electromagnetic repulsion.
- 🤔 The strong force is about 100 times stronger than the electromagnetic force, which is necessary to maintain the stability of atomic nuclei.
- 📊 The strong force has a limited range and acts like a contact force, unlike the electromagnetic force which has an infinite range.
- 🧲 The strong force is likened to Velcro, only acting when particles are in direct contact, and diminishing as they are pulled apart.
- 💡 The stability of low mass nuclei is due to the strong force's ability to overcome electromagnetic repulsion, while heavy nuclei are unstable due to the limited range of the strong force.
- 🌟 The highest stable element has no more than 100 protons, indicating the upper limit of the strong force's effectiveness.
- 🔬 The script primarily discusses nuclear physics, with a promise of a future video to connect the strong force to particle physics.
Q & A
What are the four fundamental forces mentioned in the script?
-The four fundamental forces discussed in the script are gravity, electromagnetism, the strong nuclear force, and the weak nuclear force.
Who discovered the basic structure of the atom and when?
-Ernest Rutherford discovered the basic structure of the atom in 1911, which consists of a small and dense core of positive electric charge called the nucleus, surrounded by a cloud of negatively charged electrons.
What did Ernest Rutherford discover in 1917 that contributed to our understanding of the atomic nucleus?
-In 1917, Ernest Rutherford discovered the proton, which revealed that the nucleus is made up of smaller blobs of positive charge, rather than a single large blob.
Why do protons in a nucleus repel each other?
-Protons in a nucleus repel each other because they each have a positive electrical charge, and like charges repel each other.
How much force is involved in the repulsion between two protons?
-The repulsion force between two protons is about 20 pounds, which is a significant force in the subatomic world.
What force counteracts the electromagnetic repulsion between protons in an atomic nucleus?
-The strong nuclear force counteracts the electromagnetic repulsion between protons in an atomic nucleus, holding them together.
How strong is the strong nuclear force compared to the electromagnetic force?
-The strong nuclear force is about 100 times stronger than the electromagnetic force.
What is unique about the range of the strong nuclear force compared to the electromagnetic force?
-Unlike the electromagnetic force, which has an infinite range, the strong nuclear force acts like a contact force with a much shorter range, becoming effective only when particles are in close proximity.
Why are heavy nuclei unstable according to the script?
-Heavy nuclei are unstable because the strong force, which only acts between neighboring particles, cannot overcome the repulsion of all protons pushing against each other in a large nucleus.
What is the highest number of protons found in a stable element according to the script?
-The highest stable element has no more than 100 protons.
What does the script suggest about the relationship between the strong force and particle physics?
-The script suggests that while the strong force is a topic of nuclear physics, it will be discussed further in relation to particle physics in another video.
Outlines
🔬 Fundamental Forces and the Strong Nuclear Force
This paragraph introduces the concept of fundamental forces in particle physics, focusing on the strong nuclear force. It explains that the strong nuclear force is necessary to counteract the electromagnetic repulsion between protons in an atomic nucleus. Ernest Rutherford's discovery of the atomic structure and the proton is mentioned, highlighting the repulsive force between like charges. The strong force is described as being significantly stronger than the electromagnetic force, approximately 100 times stronger, and having a much shorter range, which is crucial for the stability of low mass nuclei but not for heavier ones. The paragraph also touches on the limitations of the strong force, as seen in the periodic table, where the highest stable element has no more than 100 protons.
Mindmap
Keywords
💡Particle Physics
💡Fundamental Forces
💡Strong Nuclear Force
💡Ernest Rutherford
💡Nucleus
💡Proton
💡Electromagnetic Repulsion
💡Uranium
💡Periodic Table
💡Neutrons
💡Nuclear Physics
Highlights
Introduction to the fundamental forces in particle physics, including gravity, electromagnetism, strong nuclear force, and weak nuclear force.
Explanation of the discovery of the atom's structure by Ernest Rutherford in 1911 and the identification of the proton in 1917.
Discussion on the repulsion force between protons due to their positive electrical charges.
Quantification of the electromagnetic repulsion force between two protons as being about 20 pounds.
Illustration of the strong nuclear force as the counteracting force to the electromagnetic repulsion within the atomic nucleus.
Estimation that the strong force is approximately 100 times stronger than the electromagnetic force.
Introduction of the concept that the strong force has a limited range, unlike the infinite range of electromagnetism.
Description of the strong force as a 'contact force' that diminishes when particles are separated.
Explanation of why low mass nuclei are stable due to the strong force's properties.
Insight into why heavy nuclei are unstable, given the strong force's limited range and the repulsion among protons.
Mention of the highest stable element having no more than 100 protons, indicating the strong force's limitations.
Clarification that the presence of neutrons in the nucleus does not significantly alter the discussion on the strong force.
Introduction of the strong force as the strongest known force in the universe, with a slight correction to being the second strongest.
Promise of a future video that will delve into the strong force's role in the particle physics world.
The strong force's unique characteristics that differentiate it from the electromagnetic force.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: