Writing and Balancing Reactions Predicting Products
TLDRThe video script presents a detailed explanation on predicting chemical reaction products, focusing on identifying the type of reaction first, which simplifies the process. It emphasizes that for composition reactions, there's typically one product, while decomposition and combustion reactions have specific expected products like CO2 and H2O. The speaker guides viewers through the steps of writing down potential products, balancing charges within compounds, and ensuring the final reaction makes sense based on the initial assumptions. Examples are provided to illustrate single replacement, double replacement, and metal plus water reactions, highlighting the importance of balancing charges and understanding the periodic table's influence on element charges. The script concludes with an AP-level challenge, encouraging viewers to apply their knowledge to more complex scenarios.
Takeaways
- 🔍 **Identify Reaction Type First**: Before predicting products, determine if the reaction is composition, decomposition, single replacement, or double replacement.
- 📝 **Write Down Expected Products**: After identifying the reaction type, write down the products you expect to form based on the reactants.
- ⚖️ **Balance Charges**: Ensure that the charges within compounds are balanced, which is crucial for predicting the correct reaction products.
- 🔄 **Switch Places in Single Replacement**: In a single replacement reaction, the single element and the element in the compound switch places.
- 🔁 **First Things Switch in Double Replacement**: In a double replacement reaction, the cations and anions of the reactants switch places to form new compounds.
- 🔍 **Use Periodic Table for Charges**: Refer to the periodic table to understand the typical charges of elements, which helps in balancing compounds.
- ✅ **Double Check Your Work**: After writing the reaction, make sure it makes sense with the original assumption of the reaction type.
- 🚫 **Avoid Forgetting Hydrogen in Water**: When dealing with water in reactions, remember that it can act as H⁺ and OH⁻, and not all hydrogens are replaced in single replacement reactions.
- 🔥 **Combustion Reactions**: Recognize when a reaction with O₂ is a combustion reaction, which typically produces CO₂ and H₂O for hydrocarbons.
- 🧲 **Charges Must Balance**: In any chemical reaction, the total charge before and after the reaction must remain the same.
- 📐 **Use Common Denominators**: When balancing complex reactions, using common denominators can help to systematically balance the elements involved.
- 📚 **Practice and Review**: The script emphasizes the importance of practicing different types of chemical reactions and reviewing them to gain proficiency.
Q & A
What is the first step in predicting the products of a chemical reaction?
-The first step is to identify the type of reaction it is, as this will guide you on what to expect for the products.
What is the significance of determining the type of reaction in predicting the products?
-Knowing the type of reaction helps to narrow down the possible products. For example, in a decomposition reaction, you would expect multiple products, while in a combustion reaction involving a hydrocarbon, the products are always CO2 and H2O.
How do you balance the charges inside a compound during the prediction of reaction products?
-You balance the charges by ensuring that the total positive charge equals the total negative charge within the compound. For instance, in potassium bromate (KBr), potassium has a +1 charge and bromine has a -1 charge, balancing each other out.
What is a single replacement reaction and how does it affect the prediction of products?
-A single replacement reaction involves a single substance and a compound, where an element in the compound is replaced by the single substance. The prediction involves determining which elements will switch places and then balancing the charges to find the products.
What is a double replacement reaction and how does it differ from a single replacement reaction?
-A double replacement reaction involves two compounds exchanging parts rather than a single substance replacing an element in a compound. The prediction involves determining which parts will switch places and then ensuring the charges are balanced in the resulting compounds.
How does the position of an element in the periodic table influence its charge in a compound?
-Elements on the left side of the periodic table tend to have a positive charge, while those on the right side tend to have a negative charge. This is because elements on the left are more likely to lose electrons (be oxidized) and those on the right are more likely to gain electrons (be reduced).
What is the typical charge of iron in a compound?
-The typical charge of iron in a compound is +3.
In the context of the script, what does 'combustion' refer to and what are the expected products?
-In the context of the script, 'combustion' refers to a reaction where a hydrocarbon reacts with oxygen (O2). The expected products of a hydrocarbon combustion are carbon dioxide (CO2) and water (H2O).
What is the process of balancing charges in a compound called?
-The process of balancing charges in a compound is called charge balance or charge neutrality, ensuring that the total positive charge from all cations equals the total negative charge from all anions in the compound.
Why is it important to double-check the reaction after predicting the products?
-Double-checking the reaction is important to ensure that the charges are balanced, the reaction is consistent with the type of reaction identified, and the products make sense based on the reactants and the rules of chemistry.
How does the script suggest approaching the prediction of products for reactions involving metals and water?
-The script suggests identifying the type of reaction (single replacement in the case of metals and water), understanding that water can act as H-OH, and predicting that the metal will replace the hydrogen to form a hydroxide and release hydrogen gas.
What is the general approach to solving a chemical reaction problem as described in the script?
-The general approach involves three steps: 1) Identifying the type of reaction, 2) Writing down the expected products, and 3) Balancing the charges within the compounds to ensure the reaction is chemically feasible.
Outlines
🧪 Understanding Reactions: Predicting Products
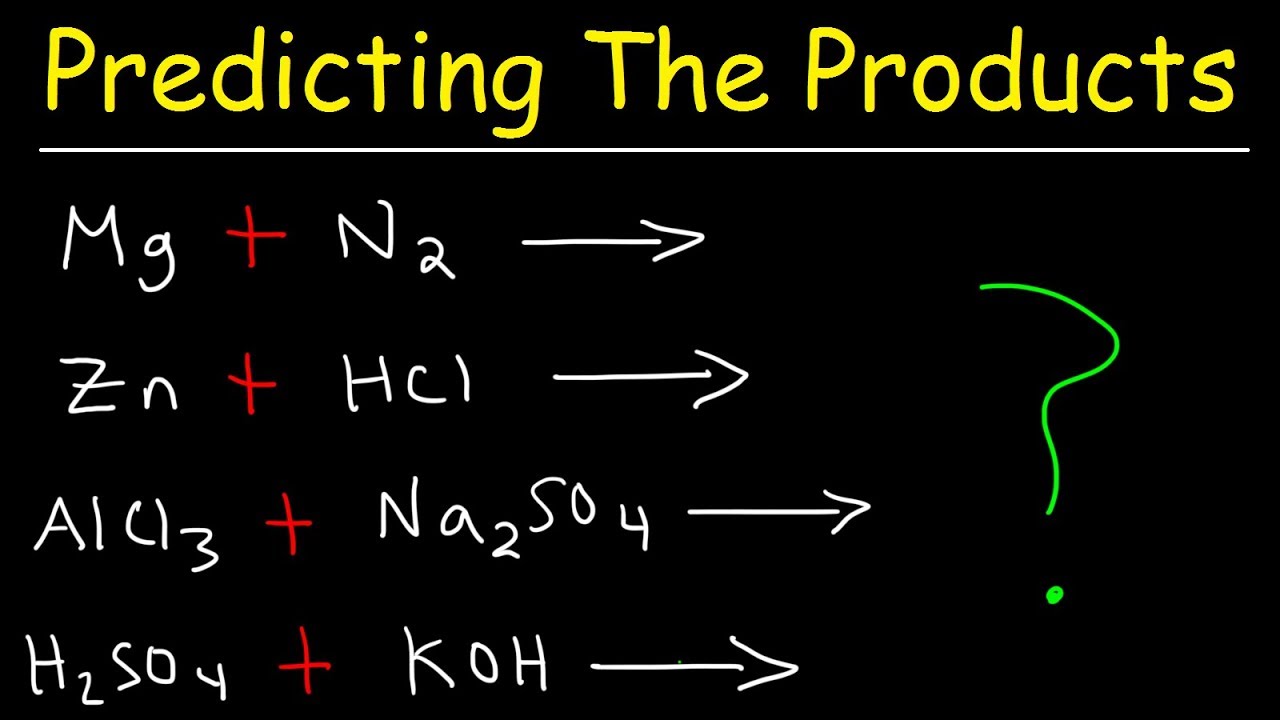
This paragraph introduces the complexity of predicting chemical reaction products. The key to solving these problems is to first identify the type of reaction, such as composition, decomposition, or combustion. Once the reaction type is known, one can predict the products more accurately. For example, in a decomposition reaction, there are multiple products, while in a composition reaction, there is typically just one product. The paragraph also emphasizes the importance of balancing charges within compounds and provides an example of a single replacement reaction with iron and copper chloride, illustrating the process of predicting the products and ensuring charge balance.
🔍 Advanced Reactions: Balancing and Types
The second paragraph delves into more advanced reaction types, focusing on double replacement reactions and the process of balancing chemical equations. It explains that in a double replacement reaction, the cations and anions of the reactants switch places to form new compounds. The paragraph provides an example with copper and silver nitrate, demonstrating how to predict and write the products of the reaction, ensuring that the charges are balanced. It also touches on the concept of combustion reactions with hydrocarbons, which always produce CO2 and H2O. The speaker encourages the audience to practice with five examples and provides solutions, emphasizing the need to identify the reaction type, predict the products, and check for balance.
🔬 AP Chemistry Level: Reactions with Water
The third paragraph raises the difficulty to the AP Chemistry level, discussing reactions involving metals and water, as well as double replacement reactions. It explains that when a metal reacts with water, it's a single replacement reaction where the metal displaces hydrogen from the water molecule. The paragraph provides an example with sodium and water, showing how to write the balanced chemical equation. Another example is given with tin chloride and iron(III) sulfate, illustrating a double replacement reaction where the metals switch places and the charges are balanced. The speaker advises starting with nonmetals when balancing such equations and emphasizes the importance of keeping track of the charges and stoichiometry in the reaction.
Mindmap
Keywords
💡Reaction Type
💡Reactants
💡Products
💡Balancing Charges
💡Single Replacement
💡Double Replacement
💡Combustion
💡Hydrocarbon
💡Metal plus Water
💡Charges in Compounds
💡AP Test
Highlights
The key to predicting reaction products is first identifying the type of reaction, such as composition, decomposition, or combustion.
For a single replacement reaction, one substance replaces another in a compound.
In a double replacement reaction, the cations and anions of the reactants switch places.
Balance the charges within compounds to ensure the reaction is correct.
Use parentheses to group elements together in complex reactions.
When balancing reactions, start with nonmetals like chlorine as they are often easier to balance.
For metal plus water reactions, the metal replaces a hydrogen atom in the water molecule, forming a hydroxide and hydrogen gas.
Water is treated as H-O-H in reactions, with the hydrogen and oxygen acting as separate entities.
In combustion reactions involving hydrocarbons, the only products are CO2 and H2O.
When balancing complex reactions, keep elements that do not change together, like sulfate.
After balancing a reaction, double check that it makes sense based on the original reaction type.
For single replacement reactions, the reactants and products should be one element/compound and one compound/element.
In a combustion reaction with O2, the products are always CO2 and H2O for hydrocarbons.
When balancing, if the charges are already balanced, you do not need to adjust them further.
For complex reactions, find a common denominator to help balance the elements more easily.
When adding charges, make sure to multiply the entire formula by the necessary number to balance it.
Remember that hydrogen gas is diatomic (H2) and balance it as such in reactions.
Always check each step of the process - identify the reaction type, write the products, and balance the charges.
Transcripts
Browse More Related Video

Predicting Products of Chemical Reactions: Practice Problems

Predicting Products | Double Replacement Reactions

Solving Chemical Reactions - Predicting the Products - CLEAR & SIMPLE CHEMISTRY
Predicting The Products of Chemical Reactions - Chemistry Examples and Practice Problems

ALEKS: Predicting the products of a single displacement reaction involving hydrogen
How To Write Net Ionic Equations In Chemistry - A Simple Method!
5.0 / 5 (0 votes)
Thanks for rating: