18.6 Nucleophilic Aromatic Substitution | Organic Chemistry
TLDRThe video script delves into nucleophilic aromatic substitution (NAS) reactions, contrasting them with electrophilic aromatic substitution (EAS) reactions. Unlike EAS, where benzene acts as a nucleophile, NAS involves benzene as an electrophile, necessitating a strong nucleophile like amide ion (NanH2), hydroxide (NaOH), or alkoxide (NaOCH3). Two primary mechanisms are discussed: addition-elimination and elimination-addition, also known as the benzyne mechanism. The former is favored by electron-withdrawing groups like nitro groups, which stabilize the carbanion intermediate through resonance, while the latter occurs in their absence. The script explains how the presence of electron-withdrawing groups influences the reactivity and mechanism, with electron-donating groups being deactivating. The lesson also touches on the regioselectivity of the reaction, leading to different products based on the mechanism. Practical implications for synthesis are briefly mentioned, with an emphasis on EAS reactions and side chain reactions being more common in synthesis problems.
Takeaways
- 🔍 Nucleophilic aromatic substitution (NAS) involves a benzene ring acting as an electrophile, in contrast to electrophilic aromatic substitution (EAS) where benzene acts as a nucleophile.
- ⚙️ Two main mechanisms for NAS are the addition-elimination mechanism and the elimination-addition (benzyne) mechanism, with the choice influenced by the presence of electron-withdrawing or donating groups.
- 🚫 Strong nucleophiles, typically with a negative charge like amide ions, hydroxide, or alkoxide, are required for NAS reactions.
- 🔁 The addition-elimination mechanism occurs in two steps: nucleophilic addition to form a carbanion, followed by elimination to expel the leaving group and restore aromaticity.
- 🔁 The elimination-addition mechanism also occurs in two steps but begins with the elimination to form a benzyne intermediate, followed by nucleophilic addition.
- 📍 The presence of electron-withdrawing groups like nitro groups (NO2) in ortho or para positions activates the benzene ring and stabilizes the carbanion intermediate, favoring the addition-elimination mechanism.
- 📍 Electron-donating groups like methyl groups deactivate the benzene ring and favor the elimination-addition mechanism when no strong electron-withdrawing groups are present.
- ➡️ The regiochemistry of the substitution can vary depending on the mechanism, leading to different possible products.
- 🧪 The type of mechanism can sometimes be inferred from the products formed in a lab setting, with two possible products indicating the elimination-addition mechanism.
- 🔬 Electron-donating groups make the benzene ring less reactive in NAS reactions, while electron-withdrawing groups increase reactivity.
- ✅ Both mechanisms can be relevant in synthesis, although electrophilic aromatic substitution (EAS) and side chain reactions are more commonly utilized in practice.
Q & A
What is the main topic of this lesson?
-The main topic of this lesson is nucleophilic aromatic substitution (NAS).
What is the difference between EAS and NAS reactions in terms of the role of the benzene ring?
-In EAS reactions, the benzene ring acts as a nucleophile, whereas in NAS reactions, the benzene ring acts as an electrophile.
What are the two possible mechanisms for nucleophilic aromatic substitution?
-The two possible mechanisms for nucleophilic aromatic substitution are the addition-elimination mechanism and the elimination-addition (benzyne) mechanism.
What type of nucleophile is typically required for a nucleophilic aromatic substitution reaction?
-A strong nucleophile with a negative charge is typically required for a nucleophilic aromatic substitution reaction, such as amide ions, hydroxide, or alkoxide.
How does the presence of electron withdrawing groups affect the likelihood of the addition-elimination mechanism in NAS reactions?
-The presence of electron withdrawing groups, particularly in the ortho or para positions, makes the addition-elimination mechanism more likely by helping to stabilize the carbanion intermediate.
What is a key difference between the reactivity of aryl halides in EAS and NAS reactions?
-In EAS reactions, electron donating groups are activating, while in NAS reactions, electron withdrawing groups are activating due to the change in the charge of the intermediate from positive in EAS to negative in NAS.
What is the name of the intermediate structure formed in the elimination-addition mechanism of NAS reactions?
-The intermediate structure formed in the elimination-addition mechanism of NAS reactions is called the benzyne.
How can you determine which mechanism has occurred in a NAS reaction if you are given the products?
-If both ortho and para substitution products are formed, it indicates that the elimination-addition mechanism has occurred. If only the para product is formed, it suggests the addition-elimination mechanism.
What is the role of the sodium ion in the nucleophilic aromatic substitution reaction using NaNH2?
-The sodium ion acts as a spectator ion during the nucleophilic aromatic substitution reaction using NaNH2, as it is the amide ion (NH2-) that acts as the strong nucleophile.
null
-null
Why are electron withdrawing groups important in stabilizing the intermediate in the addition-elimination mechanism?
-Electron withdrawing groups, such as nitro groups, are important because they can stabilize the negative charge on the carbanion intermediate by delocalizing the charge through resonance, making the reaction more favorable.
What is the significance of the regioisomer formation in the elimination-addition mechanism?
-The significance of regioisomer formation in the elimination-addition mechanism is that it allows for the possibility of two different substitution products, depending on where the nucleophile adds to the benzyne intermediate.
How does the presence of a methyl group affect the reaction mechanism?
-The presence of a methyl group, which is an electron donating group, can destabilize the carbanion intermediate, making the addition-elimination mechanism less likely and favoring the elimination-addition mechanism instead.
Outlines
🔬 Nucleophilic Aromatic Substitution (NAS) Overview
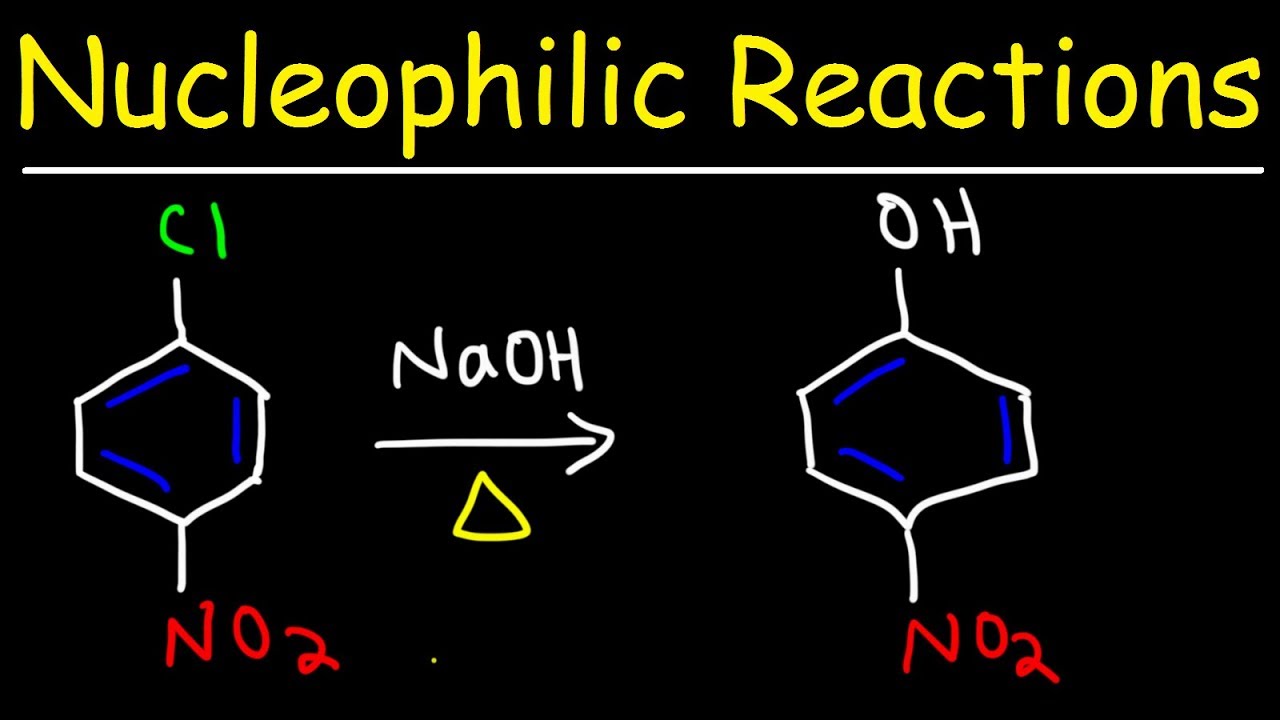
This paragraph introduces the topic of nucleophilic aromatic substitution (NAS), contrasting it with electrophilic aromatic substitution (EAS) reactions. It explains that NAS involves a benzene ring acting as an electrophile and requires a strong nucleophile, often negatively charged, such as amide ions, hydroxide, or alkoxide. The paragraph outlines two possible mechanisms for NAS: addition-elimination and elimination-addition, and hints at factors that predict which mechanism is likely to occur.
⚛️ Mechanism One: Addition-Elimination with Resonance Stabilization
The first mechanism, addition-elimination, is described with a focus on the role of electron-withdrawing groups like nitro groups in stabilizing the carbanion intermediate. The presence of these groups in ortho or para positions to the leaving group increases the reactivity of the aryl halide in NAS reactions. The paragraph also explains how electron-donating groups, unlike in EAS reactions, deactivate the benzene ring towards nucleophilic attack in NAS reactions, thus affecting the reaction mechanism and rate.
⚛️ Mechanism Two: Elimination-Addition via Benzyne Intermediate
The second mechanism, elimination-addition, is detailed with the benzyne intermediate. This pathway is favored when electron-withdrawing groups are absent, leading to the formation of a benzyne intermediate after an elimination step that involves deprotonation. The paragraph discusses how this mechanism can result in different regioisomers depending on where the nucleophile attacks the benzyne intermediate. It also touches on how the presence of electron-donating groups can affect the reaction and the importance of the position of substituents on the benzene ring.
🧪 Predicting Mechanisms and Synthesis Applications
This paragraph discusses how to predict which NAS mechanism will occur based on the presence of electron-withdrawing or donating groups and by analyzing the products formed in a lab setting. It also briefly mentions the synthetic applications of NAS reactions, noting that while they may not be as commonly encountered in academic problems as EAS reactions, they still have practical uses in the lab. The paragraph concludes with a call to action for viewers to like, share, and explore additional study materials for further learning.
Mindmap
Keywords
💡Nucleophilic aromatic substitution (NAS)
💡Electrophile
💡Nucleophile
💡Addition-elimination mechanism
💡Elimination-addition mechanism
💡Resonance stabilization
💡Benzyne intermediate
💡Electron-withdrawing groups
💡Electron-donating groups
💡Regioisomers
💡Synthesis
Highlights
Nucleophilic aromatic substitution (NAS) is a simpler reaction compared to electrophilic aromatic substitution (EAS) with fewer options and directing effects.
Two possible mechanisms for NAS reactions: addition-elimination and elimination-addition.
In NAS, the benzene ring acts as an electrophile, requiring a strong nucleophile, often with a negative charge, such as NaNH2, NaOH, or NaOCH3.
The addition-elimination mechanism involves a nucleophilic attack followed by the formation of a carbanion and elimination of the leaving group.
Resonance stabilization of the carbanion intermediate is possible with electron-withdrawing groups like NO2, especially in ortho or para positions.
Electron-withdrawing groups increase the reactivity of aryl halides in NAS reactions.
In contrast to EAS, electron-withdrawing groups are activating in NAS reactions, while electron-donating groups are deactivating.
The elimination-addition mechanism, also known as the benzyne mechanism, is more likely when electron-withdrawing groups are absent.
The benzyne intermediate is a key species in the elimination-addition mechanism, leading to different regioisomers.
The presence of electron-donating groups like a methyl group can affect the regiochemistry of the products in the elimination-addition mechanism.
The choice between the two mechanisms can often be inferred from the products formed in a lab setting.
NAS reactions are less common in synthesis problems compared to EAS and side chain reactions.
Synthetic applications of NAS reactions may include replacing halogens on a benzene ring with groups like methoxy, hydroxide, or amine.
The lecture provides insights into predicting the mechanism of NAS reactions based on the structure and reactivity of the substrates.
Electron-donating groups, such as a methyl group, can influence the regioselectivity of the reaction products.
The benzyne mechanism can lead to the formation of multiple products due to the possibility of nucleophilic attack at different sites.
Understanding the stabilizing effects of electron-withdrawing and electron-donating groups is crucial for predicting the reactivity and mechanism of NAS reactions.
Transcripts
Browse More Related Video
Nucleophilic Aromatic Substitution - Benzyne Intermediate and Meisenheimer Complex

18.1 Electrophilic Aromatic Substitution | Organic Chemistry

18.7 Retrosynthesis with Aromatic Compounds | Organic Chemistry

More EAS - Electron Donating and Withdrawing Groups: Crash Course Organic Chemistry #38

More EAS & Benzylic Reactions: Crash Course Organic Chemistry #39

Diazonium Salts & Nucleophilic Aromatic Substitution: Crash Course Organic Chemistry #47
5.0 / 5 (0 votes)
Thanks for rating: