18.1 Electrophilic Aromatic Substitution | Organic Chemistry
TLDRThe video script provides an in-depth exploration of electrophilic aromatic substitution (EAS), a key concept in the study of aromatic compounds in organic chemistry. It distinguishes EAS from nucleophilic aromatic substitution (NAS), emphasizing the role of benzene as a nucleophile in EAS, where a hydrogen is replaced by an electrophile. The script outlines the general mechanism of EAS, which involves a two-step process: nucleophilic attack by benzene on the electrophile, leading to the formation of a carbocation (referred to as a sigma complex), followed by a deprotonation step to restore aromaticity. The lesson covers the first four major EAS reactions: bromination, chlorination, sulfonation, and nitration, detailing the specific mechanisms and the role of catalysts in each. The script also discusses the unique characteristics of each reaction, such as the reversibility of sulfonation and the formation of the electrophilic species in nitration. The video aims to equip viewers with a solid understanding of EAS reactions, preparing them for further study in organic chemistry.
Takeaways
- 🔬 Electrophilic Aromatic Substitution (EAS) involves the substitution of a hydrogen atom in a benzene ring with an electrophile, while the benzene acts as a nucleophile.
- 🔁 The general mechanism for EAS consists of two main steps: nucleophilic attack on the electrophile and subsequent deprotonation to restore aromaticity.
- ⚡ Benzene's stability and low-energy electrons mean that a strong electrophile is required for a reaction to occur, which is often generated in situ.
- 🏎️ The first step in EAS, forming a carbocation, is the slow, rate-determining step due to the high activation energy required to move from an aromatic to a non-aromatic intermediate.
- 🔊 The carbocation intermediate formed in EAS is often referred to as the sigma complex and is resonance-stabilized.
- ⚖️ EAS reactions are typically catalyzed by acids, with Lewis acids like iron or aluminum being common, although some reactions use sulfuric acid as a catalyst.
- 🧪 The specific electrophile and its generation method vary for different EAS reactions, such as bromination, chlorination, sulfonation, nitration, Friedel-Crafts alkylation, and Friedel-Crafts acylation.
- 🔄 The sulfonation reaction with fuming sulfuric acid is unique as it is reversible with dilute sulfuric acid, allowing for desulfonation.
- ⏫ In nitration, nitric acid acts as a base to form a reactive electrophile, which is a nitronium ion (NO2+), and the reaction requires two steps to generate this electrophile.
- 🔵 The nitro group (NO2) in nitration has a positive formal charge on the nitrogen, which is significant for understanding its reactivity and the reaction mechanism.
- 📚 For a comprehensive understanding of EAS, it's important to learn the specific mechanisms for each type of reaction and the role of catalysts and electrophiles in these processes.
Q & A
What is the primary focus of the chapter on reactions of aromatic compounds?
-The primary focus of the chapter is on electrophilic aromatic substitution (EAS) reactions, which include bromination, chlorination, sulfonation, and nitration, as well as a discussion on side chain reactions on benzene rings and a brief introduction to nucleophilic aromatic substitution (NAS).
What is the general mechanism for electrophilic aromatic substitution?
-The general mechanism for electrophilic aromatic substitution involves two steps: nucleophilic attack of the aromatic compound on the electrophile, followed by deprotonation to restore aromaticity. This process results in the substitution of a hydrogen atom on the benzene ring with the electrophile.
Why is benzene considered stable and less reactive in EAS reactions?
-Benzene is stable and less reactive due to its aromaticity, which means its electrons are in a low-energy state and delocalization provides stability. For EAS to occur, a strong electrophile is required, which is often generated in situ from the reaction mixture.
What is the role of a Lewis acid catalyst in EAS reactions?
-A Lewis acid catalyst, such as FeBr3 or AlCl3, is used to generate a more reactive electrophile from a less reactive one. It facilitates the formation of the electrophile by coordinating with it, making it more electrophilic and thus more reactive towards the aromatic compound.
How does the sulfonation reaction differ from other EAS reactions?
-Sulfonation is unique among the EAS reactions because it is reversible with dilute sulfuric acid. The use of fuming sulfuric acid, a combination of sulfur trioxide and concentrated sulfuric acid, provides a ready-made electrophile in the form of sulfur trioxide.
What is the term used to describe the resonance-stabilized carbocation intermediate in EAS reactions?
-The resonance-stabilized carbocation intermediate in EAS reactions is often referred to as the sigma complex.
What are the two main steps involved in the formation of the electrophilic species in nitration?
-The two main steps in the formation of the electrophilic species in nitration are: 1) protonation of nitric acid by sulfuric acid, and 2) the departure of water (H2O) as a leaving group, generating a nitronium ion (NO2+) which is the electrophile.
Why is the step involving the formation of the carbocation considered the slow step in EAS reactions?
-The step involving the formation of the carbocation is considered the slow step in EAS reactions because it involves a high activation energy transition from an aromatic reactant to a non-aromatic intermediate, which is less stable and higher in energy.
What is the role of the base in the second step of the EAS mechanism?
-The role of the base in the second step of the EAS mechanism is to deprotonate a hydrogen atom from the carbocation intermediate, allowing the electrons to become pi electrons and restore aromaticity to the ring.
How does the Friedel-Crafts alkylation differ from other EAS reactions?
-Friedel-Crafts alkylation differs from other EAS reactions in that it involves the use of an alkyl halide and aluminum chloride to form a new carbon-carbon bond on the benzene ring, rather than the substitution of a hydrogen atom with an electrophile.
What is the significance of the positive formal charge on the nitrogen in the nitro group?
-The positive formal charge on the nitrogen in the nitro group (NO2) is significant because it contributes to the electrophilic nature of the group, making it more reactive in aromatic substitution reactions.
Outlines
🔬 Introduction to Electrophilic Aromatic Substitution (EAS)
The video begins with an introduction to electrophilic aromatic substitution (EAS), contrasting it with nucleophilic aromatic substitution (NAS). It emphasizes that in EAS, a hydrogen on the benzene ring is replaced by an electrophile, making benzene act as a nucleophile. The chapter will cover EAS reactions, including bromination, chlorination, sulfonation, and nitration, and will also touch on Friedel-Crafts alkylation and acylation. The general mechanism of EAS involves two steps: nucleophilic attack by the benzene ring on the electrophile, forming a carbocation, and then deprotonation to restore aromaticity. The video also mentions the importance of creating a strong electrophile, which is often done in situ, and the stability of the aromatic system.
🌟 Understanding the General Mechanism of EAS
The general mechanism for EAS is explained in detail, highlighting that it involves two main steps: nucleophilic attack by the benzene ring on an electrophile to form a carbocation (sigma complex), and a subsequent deprotonation to restore aromaticity. The video emphasizes that the first step is the slow, rate-determining step due to the high activation energy required to move from an aromatic reactant to a non-aromatic intermediate. It also discusses how the carbocation intermediate is resonance-stabilized and how substituents on the benzene ring can impact the stability of this intermediate. The video concludes by stating that all EAS reactions follow this two-step mechanism, with variations in the electrophile and base involved.
🧪 Individual EAS Reactions: Bromination and Chlorination
The video delves into the specifics of individual EAS reactions, starting with bromination and chlorination. It explains that these reactions require a catalyst, such as FeBr3 for bromination and AlCl3 (or FeCl3) for chlorination, to generate the electrophile. The mechanism involves the formation of a complex between the halide and the catalyst, resulting in a more reactive electrophile. The benzene ring then attacks this electrophile, leading to the formation of a carbocation and the regeneration of the catalyst. The video also notes that these reactions are analogous and involve the same two-step mechanism of nucleophilic attack followed by deprotonation to restore aromaticity.
⚗️ EAS Reactions: Sulfonation and Nitration
The video continues with sulfonation and nitration, two other EAS reactions. Sulfonation uses fuming sulfuric acid as the electrophile, which is unique because it is one of the few EAS reactions that is reversible. The mechanism involves nucleophilic attack on sulfur trioxide, followed by deprotonation to restore aromaticity and regenerate the sulfuric acid. Nitration, on the other hand, involves a more complex mechanism where mixed acid (a combination of nitric and sulfuric acids) is used. The video explains the protonation of nitric acid by sulfuric acid to form a reactive electrophile, which then reacts with benzene to form a nitro group. The mechanism concludes with the regeneration of the sulfuric acid catalyst.
📚 Summary and Additional Resources for EAS
The video concludes with a summary of the mechanisms for nitration and a reminder of the importance of understanding the electrophilic species and the role of the catalyst in these reactions. It also provides information on additional study materials, such as a study guide, practice quizzes, and a premium course for further learning on electrophilic aromatic substitution and other organic chemistry topics. The presenter encourages viewers to like and share the video to help others access the educational content.
Mindmap
Keywords
💡Electrophilic Aromatic Substitution (EAS)
💡Benzene
💡Electrophile
💡Catalyst
💡Resonance Stabilization
💡Sigma Complex
💡Bromination
💡Chlorination
💡Sulfonation
💡Nitration
💡Friedel-Crafts Alkylation
💡Friedel-Crafts Acylation
Highlights
Electrophilic Aromatic Substitution (EAS) involves substituting a hydrogen on a benzene ring with an electrophile.
Benzene acts as a nucleophile in EAS reactions, contrasting with Nucleophilic Aromatic Substitution (NAS) where it acts as an electrophile.
The general mechanism for EAS consists of two steps: nucleophilic attack and proton transfer to restore aromaticity.
Benzene's stability and low-energy electrons require a strong electrophile for EAS reactions to occur.
The formation of a carbocation intermediate, known as the sigma complex, is a key step in the EAS mechanism.
Resonance stabilization plays a crucial role in the stability of the carbocation intermediate in EAS reactions.
Substituents on the benzene ring can have profound impacts on the stability of the carbocation and the overall reaction.
The identity of the electrophile and the base are specific to each EAS reaction, following the general two-step mechanism.
Bromination, chlorination, sulfonation, and nitration are the four major EAS reactions covered, each with its specific mechanism.
Lewis acid catalysts, such as FeBr3 or AlCl3, are often required to generate the electrophile for EAS reactions.
Fuming sulfuric acid is used in sulfonation, and the reaction is unique for being reversible with dilute sulfuric acid.
Nitration involves a mixed acid reagent and a two-step process to generate the electrophilic species NO2+.
The Friedel-Crafts alkylation and acylation are additional EAS reactions where an alkyl or acyl group is introduced to the benzene ring.
The regeneration of the catalyst in each EAS reaction is essential, as true catalysts are not consumed in the reaction.
Protonation and deprotonation steps are critical for generating electrophiles and restoring aromaticity in EAS reactions.
Understanding the role of resonance structures and the sigma complex is vital for grasping the mechanisms of EAS reactions.
The impact of substituents on the reactivity and stability of the benzene ring is a significant consideration in EAS reactions.
The video provides a comprehensive guide to the mechanisms of EAS reactions, including the role of catalysts and the generation of electrophiles.
Transcripts
Browse More Related Video

18.6 Nucleophilic Aromatic Substitution | Organic Chemistry

Electrophilic Aromatic Substitution

22.6 EAS Reactions with Nitrogen Heterocycles | Organic Chemistry

18.7 Retrosynthesis with Aromatic Compounds | Organic Chemistry
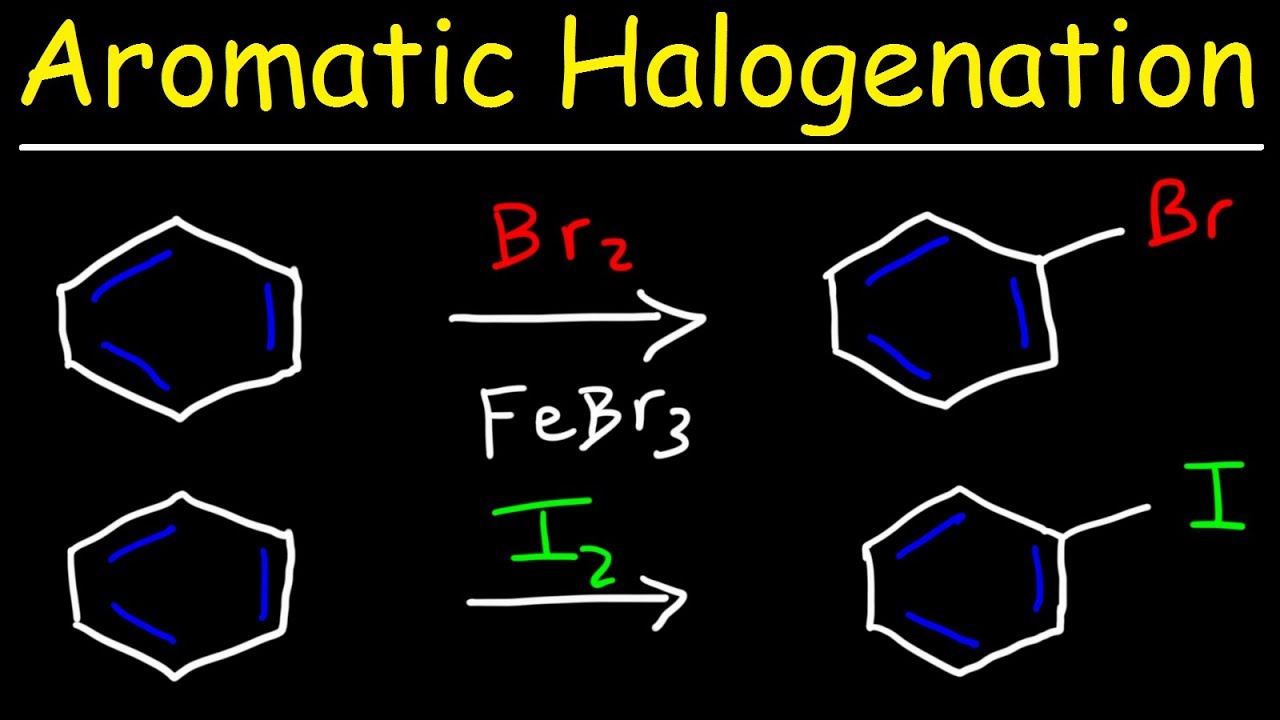
Aromatic Halogenation Mechanism - Chlorination, Iodination & Bromination of Benzene
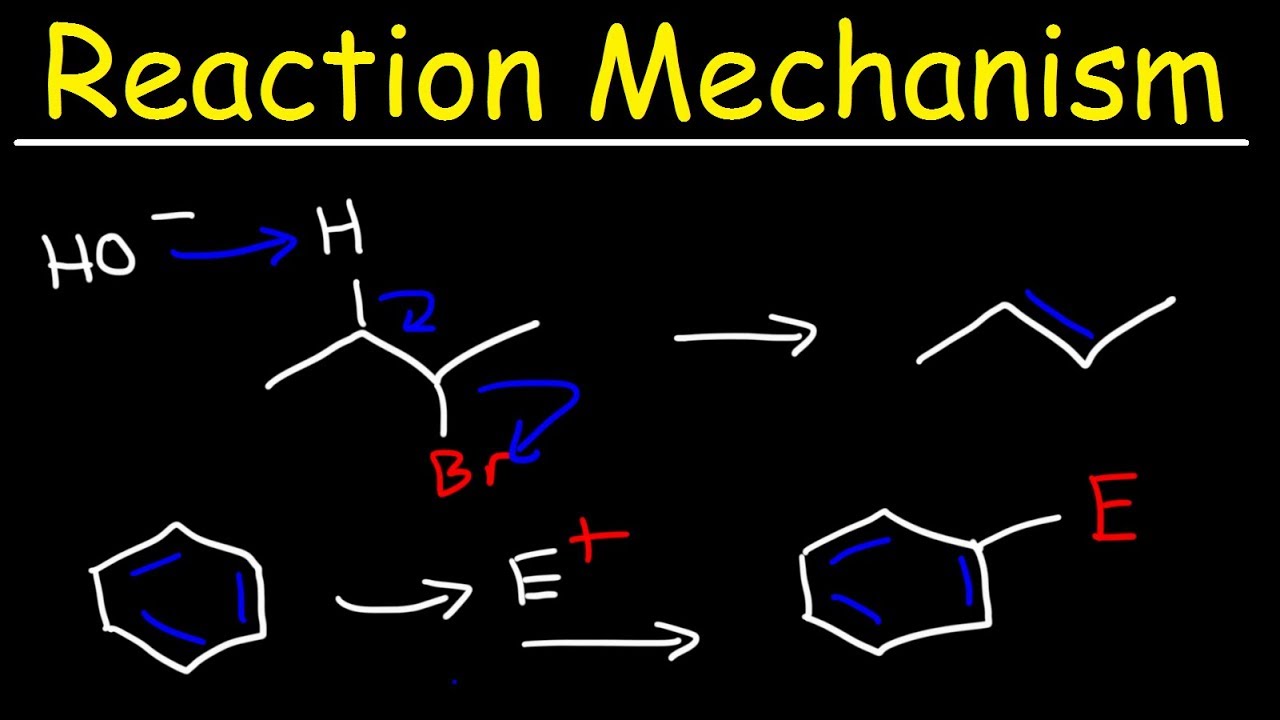
Organic Chemistry - Reaction Mechanisms - Addition, Elimination, Substitution, & Rearrangement
5.0 / 5 (0 votes)
Thanks for rating: