More EAS - Electron Donating and Withdrawing Groups: Crash Course Organic Chemistry #38
TLDRIn this episode of Crash Course Organic Chemistry, Deboki Chakravarti discusses the impact of the Chernobyl disaster on the thyroid gland due to radioactive iodine-131 and how it can lead to thyroid cancers. She explains the role of iodine in the production of thyroid hormones through electrophilic aromatic substitution (EAS) reactions. The video delves into the concept of regioselectivity in EAS reactions, focusing on how the presence of electron-donating groups (EDGs) and electron-withdrawing groups (EWGs) on a benzene ring influence the position of subsequent substitutions. Chakravarti uses examples such as nitrile and phenol to illustrate how these groups can activate or deactivate the ring and direct electrophiles to ortho, para, or meta positions. The episode also covers the influence of alkyl groups and halogens on the reactivity of benzene rings and provides a simple tool to determine whether a substituent is an EDG or EWG based on its electronegativity and connectivity. The summary emphasizes the importance of understanding these concepts for chemists aiming to synthesize complex organic molecules like thyroid hormones.
Takeaways
- 🚨 The Chernobyl disaster in 1986 led to the release of radioactive iodine-131, which contaminated the surrounding area and affected the thyroid gland in humans.
- 🧪 Thyroid hormones, such as thyroxine, are crucial for regulating metabolic rate, digestion, muscle control, and brain development, and are synthesized via electrophilic aromatic substitution (EAS) reactions.
- 💊 Doctors prescribed iodine pills containing non-radioactive iodine-127 after Chernobyl to prevent the thyroid gland from absorbing harmful radioactive iodine.
- 🔬 The regioselectivity in EAS reactions is influenced by the presence of electron-donating groups (EDGs) and electron-withdrawing groups (EWGs) on the benzene ring.
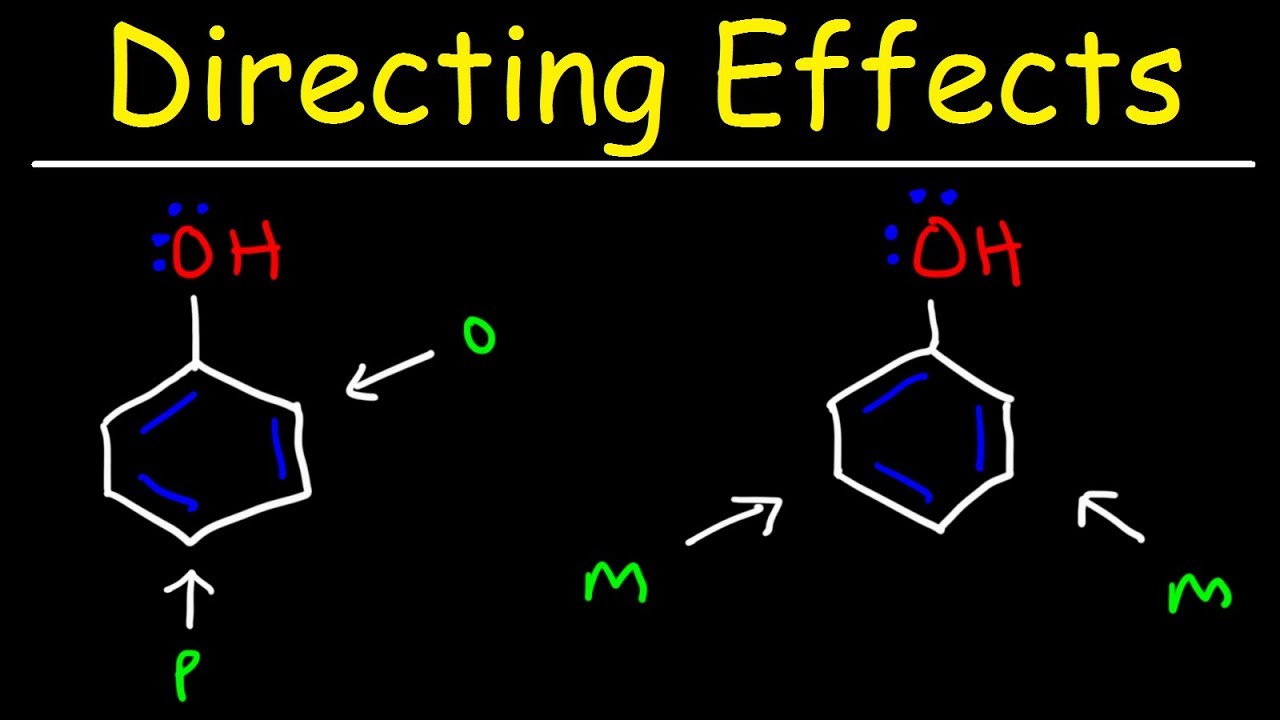
- ⏱ When multiple groups are added to a benzene ring through EAS, the positions ortho, meta, and para describe where the second group is located relative to the first group.
- 📊 EDGs activate the benzene ring, making it more nucleophilic and reactive at the ortho and para positions, while EWGs deactivate the ring and direct incoming groups to the meta position.
- 🔍 Resonance structures help determine if a group is an EDG or EWG by examining how electrons are distributed and moved around the molecule.
- 🌟 Phenol is an example of a strongly activating EDG that reacts readily with electrophiles and directs them to ortho and para positions.
- 🔬 Alkyl groups, although weakly activating, also direct electrophiles to ortho and para positions through hyperconjugation, which stabilizes charge on adjacent atoms.
- 🧲 Halogens, despite their electronegativity and deactivating inductive effect, direct electrophiles to ortho and para positions due to resonance stabilization.
- 🛠 A simple tool to determine if a substituent is EDG or EWG: if the atom bonded to the ring is connected to an atom less electronegative, it's an EDG; if more electronegative, it's an EWG.
Q & A
What major incident is mentioned at the beginning of the script related to the Chernobyl Power Plant?
-The major incident mentioned is the explosion of one of the nuclear reactors at the Chernobyl Power Plant in the north of the Ukrainian SSR in April 1986, which released a significant amount of the radioactive isotope iodine-131.
How does radioactive iodine affect the human body?
-Radioactive iodine affects the hormones produced by the thyroid gland, which are crucial for regulating the body's metabolic rate, digestion, muscle control, and brain development.
What is the term for the reaction in which iodine is incorporated into thyroid hormones?
-The reaction in which iodine is incorporated into thyroid hormones is called electrophilic aromatic substitution (EAS).
What is the role of iodine pills containing potassium iodide that were prescribed after the Chernobyl disaster?
-Iodine pills containing potassium iodide, with non-radioactive iodine-127, can be taken to temporarily satisfy the thyroid's need for iodine after exposure to radioactive iodine, thereby preventing the incorporation of the harmful radioactive isotope into the thyroid gland.
What is the term used to describe the specific positioning of groups when multiple groups are added to a benzene ring through EAS?
-The term used to describe the specific positioning of groups when multiple groups are added to a benzene ring through EAS is regioselectivity.
What are the three possible positions for the second substituent on a benzene ring when there is already one bromine present?
-The three possible positions for the second substituent are ortho (1,2-dibromobenzene), meta (1,3-dibromobenzene), and para (1,4-dibromobenzene).
How do electron donating groups (EDGs) affect the reactivity of a benzene ring?
-Electron donating groups activate the ring, making it react faster than a plain, unsubstituted benzene would. They push electrons into the ring, making it more nucleophilic and more likely to react with electrophiles in EAS reactions.
How do electron withdrawing groups (EWGs) influence the reactivity and regioselectivity of a benzene ring?
-Electron withdrawing groups deactivate the ring, making it less susceptible to reaction than plain benzene. They pull electrons from the ring, making it less nucleophilic and less likely to react with electrophiles, which makes reactions slower. They also direct incoming groups to the meta position.
What is the significance of resonance structures in determining whether a group is an EDG or EWG?
-Resonance structures help to visualize the distribution of electrons within a molecule, which can indicate whether a group is electron-donating or electron-withdrawing. By examining the movement of electrons in these structures, one can predict the group's effect on the benzene ring's reactivity and regioselectivity.
What is the role of hyperconjugation in the context of alkyl groups as EDGs?
-Hyperconjugation allows alkyl groups to donate electron density, which means the C-H bonds can stabilize charge on adjacent atoms. This makes the ortho and para positions on the benzene ring more electron-rich and thus more favorable for electrophilic attack.
How do halogens, with their lone pairs, affect the benzene ring's reactivity and regioselectivity?
-Halogens deactivate the ring due to inductive effects, but due to their resonance structures, they direct electrophiles to the ortho and para positions, albeit reluctantly, as these positions end up with negative charges that are less favorable for electrophilic attack.
What is the simple tool or rule of thumb for determining whether a substituent on a benzene ring is an EDG or EWG?
-If the atom directly bonded to the ring is connected to an atom less electronegative than itself, the substituent is electron-donating, activating, and ortho-para directing. If it's connected to an atom more electronegative than itself, the substituent is electron-withdrawing, deactivating, and meta directing.
Outlines
🔬 Chernobyl Disaster and Thyroid Cancer: The Role of Iodine
The first paragraph introduces the topic of the video, which is the study of organic chemistry through the lens of the Chernobyl disaster. It discusses the release of radioactive iodine-131 from the Chernobyl nuclear reactor explosion and its impact on the thyroid gland's hormone production. The paragraph explains how the thyroid gland uses iodine in an electrophilic aromatic substitution (EAS) reaction to produce thyroid hormones like thyroxine, which are vital for various bodily functions. It also covers the protective measures taken after the disaster, such as the use of potassium iodide pills to prevent the absorption of radioactive iodine by the thyroid. The focus then shifts to the importance of understanding regioselectivity in EAS reactions for the synthesis of thyroid hormones in a lab setting.
🌟 Understanding Regioselectivity in Electrophilic Aromatic Substitution
The second paragraph delves into the specifics of regioselectivity during electrophilic aromatic substitution reactions. It explains the concept of counting around a benzene ring to predict where a second group will attach when added through EAS. The paragraph outlines the three possible positions for a second substituent: ortho, meta, and para, and how these positions relate to the chemical behavior of the molecule. The discussion then moves to the influence of electron donating groups (EDGs) and electron withdrawing groups (EWGs) on the reactivity of the benzene ring and the directionality of incoming groups. The paragraph further explores how inductive effects from these groups determine the reactivity and regioselectivity of the EAS reaction, using examples such as the nitrile group and phenol to illustrate these concepts.
📚 Predicting Reaction Outcomes with EDGs, EWGs, and Resonance Structures
The third paragraph provides a tool for determining whether a substituent on a benzene ring is electron-donating or electron-withdrawing, and its effect on the reaction's regioselectivity. It explains that a positive charge on the substituent indicates an electron-withdrawing group that directs incoming groups to the meta position, while a connection to a less electronegative atom suggests an electron-donating group that activates the ring and directs groups to the ortho and para positions. The paragraph also discusses exceptions to this rule, such as halogens, and provides a strategy for predicting the stability of different possible reaction outcomes by drawing resonance structures. The video concludes with a teaser for the next episode, which will continue the discussion on EAS reactions and introduce benzylic reactions.
Mindmap
Keywords
💡Electrophilic Aromatic Substitution (EAS)
💡Radioactive Iodine
💡Thyroid Gland
💡Thyroxine
💡Electron Donating Groups (EDGs)
💡Electron Withdrawing Groups (EWGs)
💡Regioselectivity
💡Inductive Effects
💡Resonance Structures
💡Hyperconjugation
💡Steric Hindrance
Highlights
The Chernobyl disaster in 1986 released radioactive iodine-131, which affected the thyroid gland and could lead to thyroid cancers.
Thyroid hormones like thyroxine are synthesized through electrophilic aromatic substitution (EAS) reactions.
Iodine pills containing non-radioactive iodine-127 can prevent the incorporation of harmful radioactive iodine into the thyroid gland.
EAS reactions have specific regioselectivity when adding multiple groups to a benzene ring.
Electron donating groups (EDGs) activate the benzene ring and direct incoming groups to ortho and para positions.
Electron withdrawing groups (EWGs) deactivate the benzene ring and direct incoming groups to the meta position.
Inductive effects and resonance structures help determine if a group is an EDG or EWG.
The nitrile group is an EWG that deactivates the ring and directs electrophiles to the meta position.
Phenol is an EDG that activates the ring and directs electrophiles to ortho and para positions, leading to tribromophenol formation.
Alkyl groups are weakly activating and direct electrophiles to ortho and para positions due to hyperconjugation.
Halogens deactivate the ring due to inductive effects but still direct electrophiles to ortho and para positions due to resonance.
A simple tool to determine EDGs and EWGs involves looking at the formal charge and electronegativity of the atoms involved.
EDGs with a positive charge or connected to a less electronegative atom activate and direct to ortho-para positions.
EWGs connected to a more electronegative atom deactivate and direct to the meta position.
Exceptions to this pattern include halogens and another group that will be discussed in a future episode.
Understanding the regioselectivity of EAS reactions is crucial for synthesizing complex organic molecules like thyroid hormones.
The impact of EDGs and EWGs on reaction rates and product formation can be predicted using inductive effects and resonance structures.
Crash Course Organic Chemistry provides a comprehensive overview of EAS reactions and their implications for organic synthesis.
Transcripts
Browse More Related Video

More EAS & Benzylic Reactions: Crash Course Organic Chemistry #39
Ortho Meta Para Directors - Activating and Deactivating Groups

18.6 Nucleophilic Aromatic Substitution | Organic Chemistry

Nucleophiles and Electrophiles: Crash Course Organic Chemistry #12

Synthesis, Distillation, & Recrystallization: Crash Course Organic Chemistry #40

Diazonium Salts & Nucleophilic Aromatic Substitution: Crash Course Organic Chemistry #47
5.0 / 5 (0 votes)
Thanks for rating: