13.3 Reactions of Ethers | Organic Chemistry
TLDRThe video script focuses on the chemistry of ethers, particularly crown ethers and their role in solvating alkali metal cations, which is crucial for certain reactions like SN2. Crown ethers are cyclic polyethers named for their ability to 'crown' metal cations; their size determines the cation they can accommodate. The script explains how 12-crown-4 is suitable for lithium ions, 15-crown-5 for sodium ions, and 18-crown-6 for potassium ions, enhancing the solubility of fluoride salts in low polarity solvents. Additionally, the lesson covers the acid cleavage of ethers, a reaction where ethers, when exposed to strong acids like HCl, HBr, or HI, undergo substitution leading to the formation of alkyl halides and water. The mechanism involves protonation of the ether, forming a carbocation, which then reacts with the halide ion. A special case discussed is phenylether, where the carbon-oxygen bond adjacent to an sp2 hybridized carbon (as in a benzene ring) cannot be broken due to the instability of the resulting carbocation, leading to the formation of phenol and an alkyl halide upon reaction with hydrohalic acid.
Takeaways
- 🧪 The primary reaction of ethers is their acidic cleavage, which is not very common due to ethers being relatively inert.
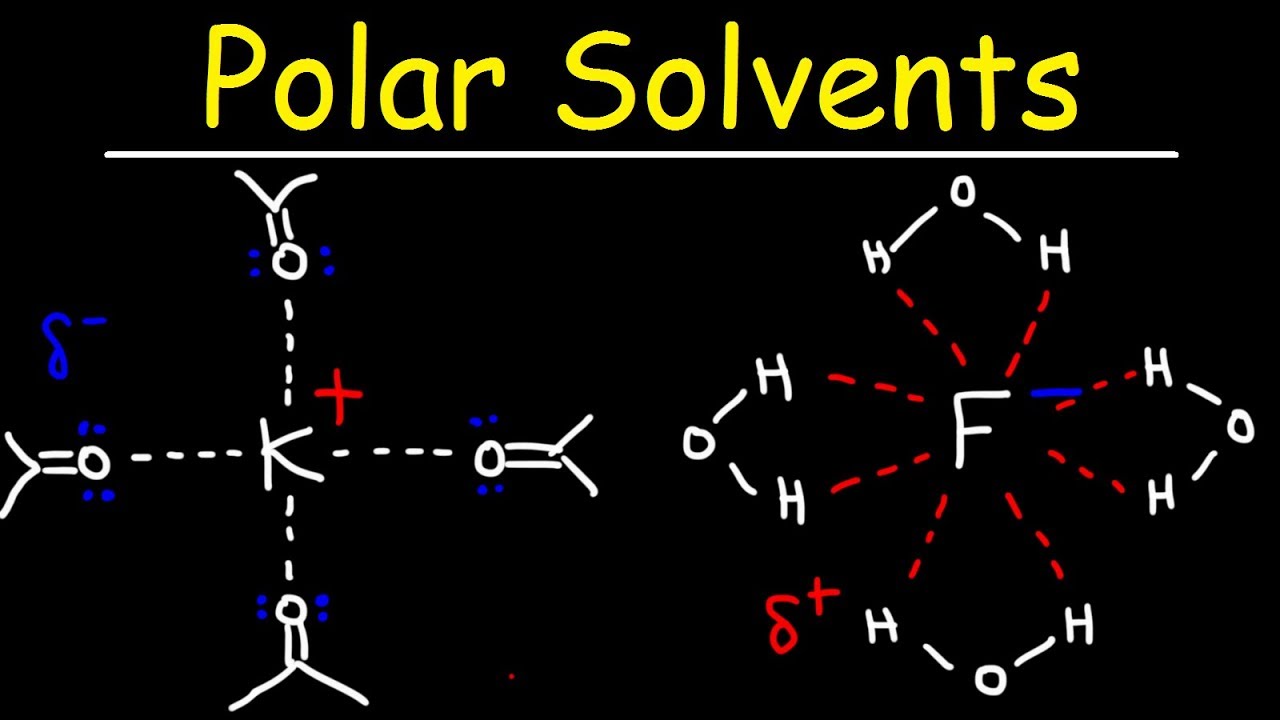
- 👑 Crown ethers are cyclic polyethers that can solvate alkali metal cations, with their size correlating to the size of the cation they can accommodate.
- 🔢 Crown ethers are named by the total number of atoms in the ring structure followed by 'crown' and the number of oxygen atoms.
- 🧲 Crown ethers increase the solubility of ionic fluoride salts in low polarity solvents, which is crucial for certain SN2 reactions.
- 💧 Acidic cleavage of ethers involves protonation, leading to the formation of alkyl halides and water.
- ⚙️ The mechanism of ether cleavage prefers SN1 when possible, but will proceed via SN2 if the adjacent carbon is primary or methyl.
- 🏃 The cleavage process starts with the formation of a carbocation at the more substituted (tertiary or secondary) carbon.
- 🔁 Potential rearrangement of the carbocation may occur, but it is not a concern in the context of this lesson.
- 🎯 The bromide ion attacks the carbocation to form the alkyl halide product.
- 🚫 Phenylethers cannot undergo cleavage at the carbon-oxygen bond adjacent to the benzene ring due to the stability of the sp2 hybridized carbon.
- ⛔ Only one equivalent of hydrohalic acid reacts with phenylethers, resulting in phenol and an alkyl halide, not two alkyl halides as with other ethers.
Q & A
What is the main topic of this lesson?
-The main topic of this lesson is the reaction of ethers, specifically focusing on crown ethers and the acidic cleavage of ethers.
What are crown ethers?
-Crown ethers are large, cyclic polyethers that resemble crowns and are capable of solvating alkali metal cations. Their size allows them to accommodate different sizes of cations.
How are crown ethers named?
-Crown ethers are named by stating the total number of atoms in the ring structure, followed by the word 'crown' and the number of oxygen atoms.
Why are crown ethers useful in certain chemical reactions?
-Crown ethers are useful in reactions where fluoride or other ionic salts need to be soluble in low polarity solvents. They solvate metal cations, allowing the anions to participate in the reaction as naked, strong nucleophiles.
What is the major reaction that ethers undergo?
-The major reaction that ethers undergo is the acidic cleavage, where they react with strong acids like HCl, HBr, or HI to break the carbon-oxygen bonds, resulting in two alkyl halides.
What is the first step in the acidic cleavage of ethers?
-The first step in the acidic cleavage of ethers is protonation, which converts the ether into a better leaving group, a neutral oxygen.
How does the mechanism of acidic cleavage of ethers differ based on the carbon type adjacent to the oxygen?
-If the adjacent carbon is tertiary or secondary, the reaction proceeds via an SN1 mechanism. If it's primary or methyl, the reaction follows an SN2 mechanism.
What happens when phenylethers undergo acidic cleavage?
-Phenylethers can only undergo acidic cleavage on one side, as the carbon-oxygen bond adjacent to the benzene ring (sp2 hybridized carbon) cannot be broken due to the instability of the resulting carbocation.
What are the products of the acidic cleavage of phenylethers?
-The products of the acidic cleavage of phenylethers are one equivalent of phenol and an alkyl halide on the other side where the carbon-oxygen bond is broken.
What is the role of the halogen in the acidic cleavage of ethers?
-The halogen from the strong acid (HX where X is a halogen) acts as a nucleophile that attacks the carbocation formed after the protonated ether leaves, leading to the formation of alkyl halides.
Why are ethers considered good solvents?
-Ethers are considered good solvents because they are relatively inert and do not readily participate in many reactions, making them suitable for a wide range of organic reactions.
Outlines
🧪 Introduction to Ether Reactions and Crown Ethers
This paragraph introduces the topic of ether reactions, focusing on the limited number of reactions ethers undergo. It emphasizes the discussion on crown ethers, which are cyclic polyethers that can solvate alkali metal cations. The size of the crown ether corresponds to the size of the cation it can solvate, with examples provided for lithium (12-crown-4), sodium (15-crown-5), and potassium (18-crown-6). The utility of crown ethers is illustrated through their use in increasing the solubility of fluoride salts in low polarity solvents, which is crucial for SN2 reactions. The paragraph also mentions that the lessons are part of an organic chemistry playlist released weekly throughout the school year.
🔍 Acid Cleavage of Ethers and Phenyl Ether Special Case
The second paragraph delves into the acid cleavage of ethers, a reaction where ethers are treated with strong acids like HCl, HBr, or HI, leading to the substitution reaction and breaking of carbon-oxygen bonds. This results in the formation of two alkyl halides and water. The mechanism involves protonation of the ether to form a good leaving group, followed by the formation of a carbocation and subsequent nucleophilic attack by a halide ion. The paragraph also discusses the SN1 and SN2 mechanisms, depending on the carbon's substitution. A special case of phenyl ethers is highlighted, where the carbon-oxygen bond adjacent to an sp2 hybridized carbon (as in a benzene ring) cannot be broken due to the inability to form a stable carbocation, resulting in the retention of the phenol group and the formation of only one alkyl halide. The paragraph concludes with a call to action for viewers to like, share, and explore additional study materials on the provided website.
Mindmap
Keywords
💡Ether
💡Crown ethers
💡Acidic cleavage
💡SN2 reaction
💡Alkali metal cations
💡Organic chemistry
💡Solubility
💡Carbocation
💡Phenyl ether
💡Hydrohalic acids
💡SN1 and SN2 mechanisms
Highlights
Ether reactions are limited, with the primary focus being on crown ethers and their unique ability to solvate alkali metal cations.
Crown ethers are cyclic polyethers that resemble crowns and vary in size to accommodate different cations.
The size of crown ethers is determined by the total number of atoms in the ring structure and the number of oxygen atoms.
12-crown-4 is the optimal size to solvate lithium ions, while 15-crown-5 and 18-crown-6 are used for sodium and potassium ions, respectively.
Crown ethers are particularly useful in SN2 reactions where they increase the solubility of fluoride salts in low polarity solvents.
The use of 18-crown-6 is necessary for solvating potassium in potassium fluoride, facilitating its use in SN2 reactions.
Ether solvents are generally unreactive, with the exception of undergoing acid cleavage in strong acids like HCl, HBr, or HI.
Acid cleavage of ethers results in the formation of two alkyl halides and water through a substitution reaction.
Protonation of the ether is the initial step in the acid cleavage mechanism, allowing for better leaving group potential.
The mechanism prefers SN1 over SN2 when possible, with the nature of the adjacent carbon determining the reaction pathway.
In the case of a secondary carbon, the reaction proceeds via SN1 to form a carbocation, which then reacts with the halide ion.
Primary alcohols undergo SN2 substitution, leading to the formation of a new alkyl halide and water.
Phenylethers exhibit unique behavior where the carbon-oxygen bond adjacent to the phenyl group cannot be cleaved due to the stability of sp2 hybridized carbons.
Phenylethers can only react with one equivalent of hydrohalic acid, resulting in the formation of phenol and an alkyl halide.
The inability to cleave the carbon-oxygen bond in phenylethers is due to the lack of a suitable leaving group and the instability of the resulting carbocation.
The lesson provides a comprehensive overview of ether reactions, focusing on the utility of crown ethers and the acid cleavage mechanism.
The practical application of crown ethers in solubilizing ionic compounds in organic solvents is highlighted, emphasizing their importance in chemical reactions.
The lesson concludes with a reminder of the limitations of acid cleavage in phenylethers, reinforcing the importance of understanding molecular structure in predicting reaction outcomes.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: