Polar Protic Solvents and Polar Aprotic Solvents For SN1 & SN2 Reactions
TLDRThis video script delves into the distinction between protic and aprotic solvents, their roles in SN1 and SN2 reactions, and how they influence reaction rates. Protic solvents like water stabilize carbocations and transition states in SN1 reactions, increasing their rates, while aprotic solvents such as acetone and crown ethers enhance SN2 reactions by not solvating anions, thus maintaining the nucleophile's reactivity. The script clarifies why SN1 reactions are favored in protic environments and SN2 in aprotic ones, providing a clear understanding of solvent effects on nucleophilic substitution reactions.
Takeaways
- 🧪 Protic solvents, such as water, methanol, and ethanol, are commonly used in SN1 and SN2 reactions and favor SN1 reactions.
- 🌡 Polar protic solvents help in the ionization process of alkyl halides by breaking the carbon-halogen bond, facilitating the SN1 reaction.
- 🔬 The ionization step in an SN1 reaction is endothermic, requiring energy input, which is provided by the solvent's interaction with the reactants.
- 🛡 Protic solvents like water can stabilize the carbocation intermediate and the halide ion formed during the SN1 reaction, lowering the activation energy.
- 🔄 The transition state in an SN1 reaction can be stabilized by polar protic solvents, which increases the reaction rate by reducing the energy barrier.
- 🌌 Polar aprotic solvents, such as acetone and dimethyl sulfoxide (DMSO), do not solvate anions and thus do not weaken the nucleophile, favoring SN2 reactions.
- 🚫 Protic solvents weaken the nucleophile strength in SN2 reactions by solvating it, which decreases the reaction rate.
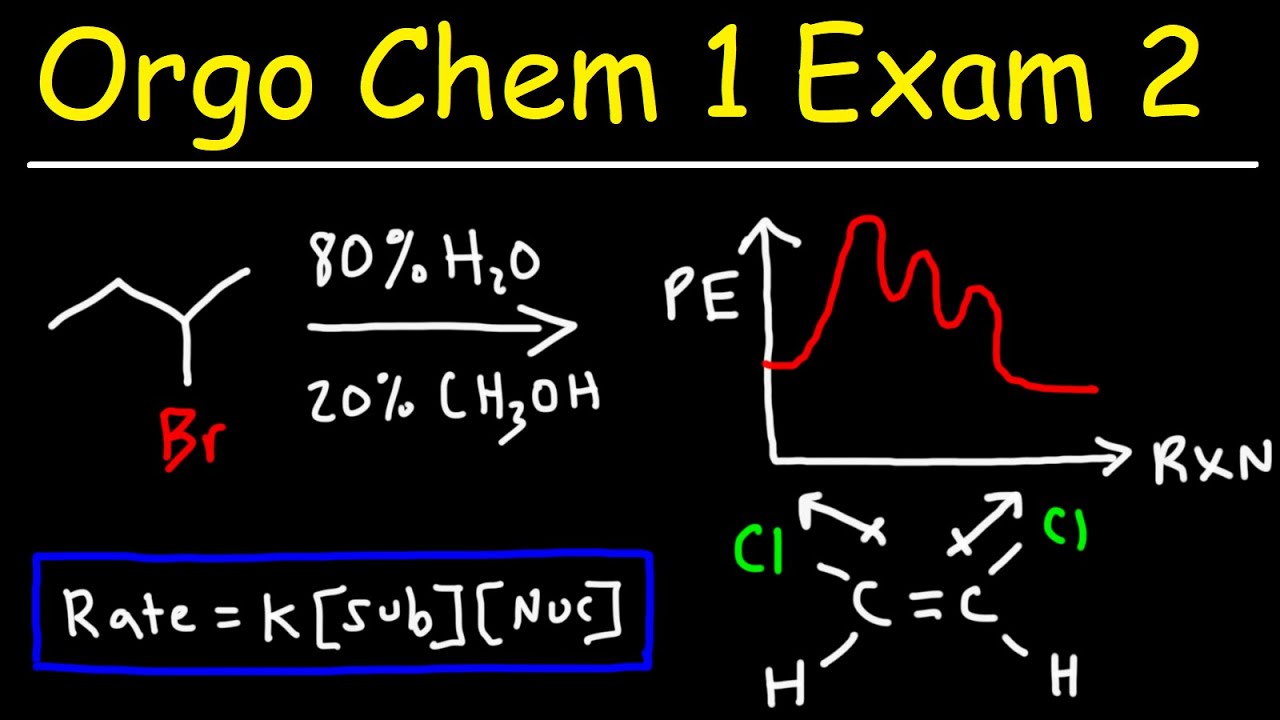
- 🔑 The rate of an SN2 reaction depends on the concentration of the substrate and the nucleophile, which is enhanced by polar aprotic solvents.
- 🏵 Crown ethers are an example of polar aprotic solvents that can solvate cations, leaving the anion (nucleophile) free to react, thus increasing the SN2 reaction rate.
- 📉 The strength of the nucleophile does not affect the rate of an SN1 reaction, as it is not included in the rate law expression.
- 📈 Polar aprotic solvents enhance the SN2 reaction rate by not solvating the nucleophile, allowing it to remain reactive and increasing the reaction's efficiency.
Q & A
What are protic solvents?
-Protic solvents are solvents that can form hydrogen bonds due to the presence of hydrogen atoms bonded to electronegative atoms like oxygen or nitrogen. Common examples include water, methanol, and ethanol.
How do protic solvents affect SN1 reactions?
-Protic solvents favor SN1 reactions by helping to ionize the alkyl halide into a carbocation and a halide ion. They can also stabilize the carbocation intermediate and the transition state, thereby lowering the activation energy and increasing the reaction rate.
What is the role of the solvent in the ionization step of an SN1 reaction?
-The solvent, particularly a polar protic one like water, assists in the ionization step of an SN1 reaction by pulling apart the carbon-halogen bond through electrostatic forces, thus facilitating the formation of a carbocation.
How do polar protic solvents stabilize carbocations?
-Polar protic solvents stabilize carbocations by solvating them through the interaction of the solvent's oxygen atoms with the positively charged carbon atom of the carbocation.
What is an aprotic solvent and what are some examples?
-Aprotic solvents are polar solvents that do not have hydrogen atoms bonded to highly electronegative atoms, thus they cannot form hydrogen bonds. Examples include acetonitrile, acetone, dimethyl sulfoxide (DMSO), and crown ethers.
Why do aprotic solvents favor SN2 reactions?
-Aprotic solvents favor SN2 reactions because they do not solvate the nucleophile, allowing it to remain reactive and increasing the likelihood of a bimolecular collision with the substrate.
How does solvation of the nucleophile by a protic solvent affect the SN2 reaction rate?
-Protic solvents, by solvating the nucleophile, effectively 'trap' it, reducing its reactivity and thus decreasing the rate of the SN2 reaction.
What is the significance of the transition state in the SN1 reaction and how is it stabilized?
-The transition state in an SN1 reaction is the point at which the carbon-halogen bond is breaking and the carbocation is forming. It is stabilized by polar protic solvents, which lower the activation energy and increase the reaction rate.
How does the solvation of ions by water affect the SN1 and SN2 reactions differently?
-In SN1 reactions, water stabilizes the carbocation and the transition state, promoting the reaction. In SN2 reactions, water solvates the nucleophile, reducing its reactivity and thus slowing down the reaction.
Why are nonpolar solvents not suitable for SN2 reactions involving ionic compounds?
-Nonpolar solvents are not suitable for SN2 reactions involving ionic compounds because these ionic compounds do not dissolve well in nonpolar solvents due to the lack of polarity to interact with the ions.
What is the role of a crown ether in SN2 reactions?
-A crown ether acts as a polar aprotic solvent in SN2 reactions by solvating the cation (e.g., potassium ion in potassium fluoride) and leaving the anion (e.g., fluoride ion) free to act as a nucleophile, thus enhancing the SN2 reaction rate.
Outlines
🧪 Protic and Aprotic Solvents in SN1 and SN2 Reactions
This paragraph introduces the concepts of protic and aprotic solvents and their roles in SN1 and SN2 reactions. Protic solvents, which contain hydrogen bonds, such as water, methanol, and ethanol, favor SN1 reactions. In contrast, polar aprotic solvents, which lack OH or NH groups, like acetyl nitrate, acetone, dimethyl sulfoxide (DMSO), and crown ethers, favor SN2 reactions. The paragraph explains how protic solvents facilitate and stabilize the ionization process in SN1 reactions, leading to the formation of a carbocation and a halide ion, and how they stabilize the transition state, thereby lowering the activation energy and increasing the reaction rate.
🌡 Stabilization of Ions and Transition States in SN1 Reactions
This section delves into the specifics of how protic solvents stabilize ions and transition states during SN1 reactions. It describes the endothermic process of breaking the carbon-bromine bond in an alkyl halide and the exothermic process of forming ion-dipole interactions that stabilize the carbocation. Protic solvents like water are shown to solvate both the carbocation and the halide ion, reducing the activation energy by stabilizing the transition state. The energy diagram illustrates how stabilization of the transition state can increase the reaction rate, making SN1 reactions more favorable in protic environments.
🚀 Enhancing SN2 Reactions with Polar Aprotic Solvents
The final paragraph contrasts the effects of polar aprotic solvents on SN2 reactions with those of protic solvents. It explains that the rate of an SN2 reaction is dependent on the substrate and nucleophile concentrations. Polar aprotic solvents, such as acetone and crown ethers, are shown to enhance the SN2 reaction by not solvating the nucleophile, thus maintaining its reactivity. The paragraph also discusses how these solvents solvate cations but not anions, allowing the nucleophile to freely react with the alkyl halide and increase the reaction rate. The summary underscores the importance of solvent choice in determining the efficiency of SN1 and SN2 reactions.
Mindmap
Keywords
💡Protic Solvent
💡Aprotic Solvent
💡SN1 Reaction
💡SN2 Reaction
💡Carbocation
💡Nucleophile
💡Leaving Group
💡Ionization
💡Activation Energy
💡Solvate
💡Endothermic Process
Highlights
Protic solvents, such as water, methanol, and ethanol, favor SN1 reactions due to their ability to form hydrogen bonds.
Aprotic solvents, like acetyl nitrate, acetone, and dimethyl sulfoxide (DMSO), favor SN2 reactions as they lack OH or NH groups.
In SN1 reactions, the ionization of an alkyl halide is an endothermic process requiring energy input.
Protic solvents facilitate the ionization of alkyl halides by interacting with the leaving group and the carbon atom.
Water can stabilize carbocations and bromide ions formed during SN1 reactions through solvation.
The stabilization of the transition state by protic solvents lowers the activation energy and increases the reaction rate in SN1 reactions.
Polar aprotic solvents enhance the strength of nucleophiles in SN2 reactions by not solvating them, unlike protic solvents.
Protic solvents weaken the nucleophile strength by solvating it, which decreases the rate of SN2 reactions.
Aprotic solvents are essential for SN2 reactions as they solvate cations but not anions, keeping the nucleophile reactive.
Crown ethers, a type of polar aprotic solvent, are effective in solvating potassium ions, freeing fluoride ions for SN2 reactions.
Protic solvents are beneficial for SN1 reactions as they stabilize the transition state and the carbocation intermediate.
The rate of SN1 reactions depends solely on the substrate and is unaffected by the nucleophile's strength.
In contrast, the rate of SN2 reactions is dependent on both the substrate and the nucleophile's concentration.
Polar aprotic solvents increase the nucleophile's reactivity, thus increasing the rate of SN2 reactions.
Nonpolar solvents are not suitable for SN2 reactions involving ionic compounds like potassium fluoride.
The choice of solvent in a reaction can significantly impact the reaction rate and mechanism.
Understanding the properties of protic and aprotic solvents is crucial for predicting and controlling reaction outcomes.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: