Intro to Electrophilic Aromatic Substitution: Crash Course Organic Chemistry #37
TLDRThe video script from Crash Course Organic Chemistry, hosted by Deboki Chakravarti, delves into the chemistry of benzene and its derivatives. It explains that polystyrene, a common plastic material, is made from styrene monomers. The video explores the unique reactivity of benzene due to its delocalized electrons, which prevents it from undergoing typical alkene addition reactions. Instead, benzene undergoes electrophilic aromatic substitution, a process that involves a catalyst and the formation of a carbocation intermediate. The script covers various substitution reactions, including bromination, chlorination, and nitration, leading to compounds like nitrobenzene, which has diverse industrial applications but also poses health risks. The episode also highlights the significance of the Friedel-Crafts alkylation and acylation reactions, which allow the formation of carbon-carbon bonds on benzene rings, creating a variety of important chemicals for the chemical industry. The video emphasizes the importance of understanding these reactions for synthetic organic chemistry.
Takeaways
- 🛍️ Polystyrene, a common packaging material, is made from styrene monomers.
- 🔬 Benzene, a key component in many substances, is primarily sourced from crude oil and can be modified through chemical reactions.
- ⚙️ Dehydrogenation is an oxidation reaction that involves attaching an ethyl group to benzene and removing hydrogens and electrons.
- 🔗 The formation of carbon-carbon bonds is a fundamental process in the chemical industry for attaching various groups to benzene rings.
- 🚫 Unlike alkenes, benzene does not readily undergo addition reactions without a catalyst due to its delocalized electrons and lack of true double bonds.
- 🔮 Electrophilic aromatic substitution is a reaction where a hydrogen on a benzene ring is replaced by an electrophile, maintaining the aromatic pi system.
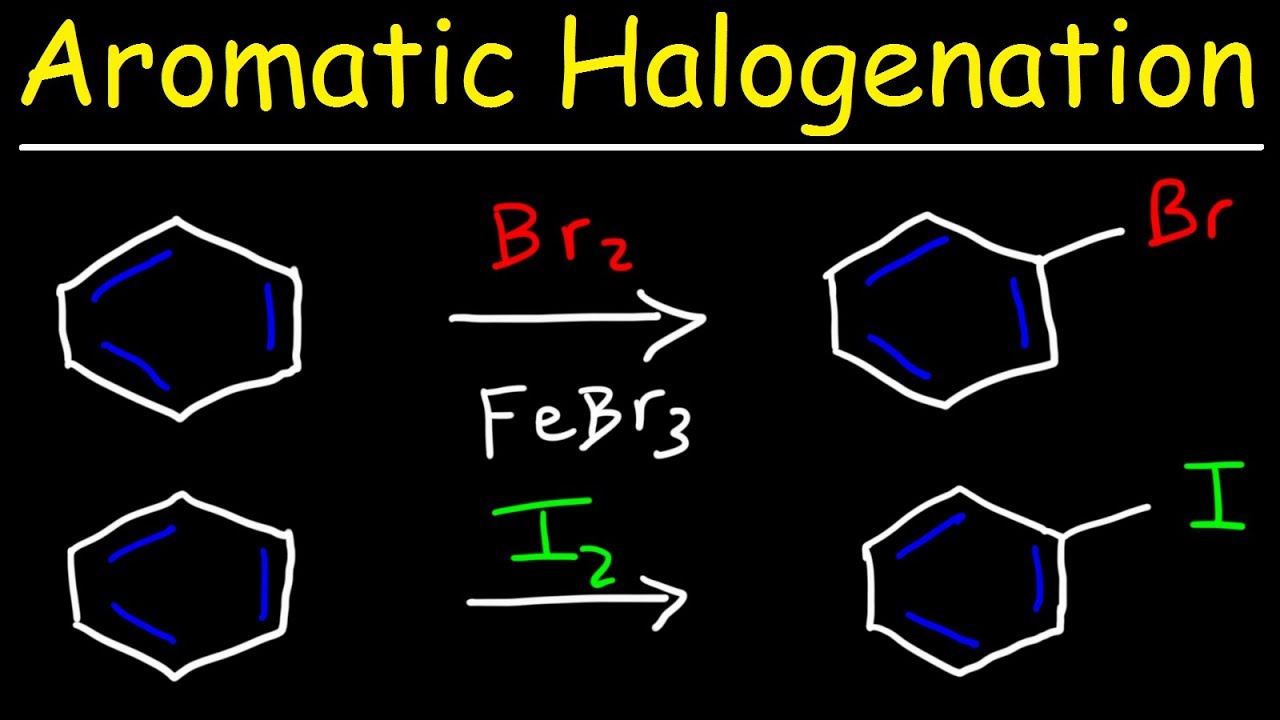
- 🧲 Lewis acids, such as ferric bromide, can polarize bromine to make it a better electrophile for reactions with benzene.
- ⚒️ Nitration and sulfonation are examples of aromatic substitution reactions where specific functional groups replace hydrogens on the benzene ring.
- 🧪 Nitrobenzene, a product of benzene nitration, has various industrial uses but also poses health risks and is potentially carcinogenic.
- 🔬 Friedel-Crafts alkylation and acylation are significant reactions that allow the formation of carbon-carbon bonds on benzene rings, leading to the synthesis of a wide range of chemicals.
- ⚠️ Carbocation rearrangement can occur during Friedel-Crafts reactions, leading to the formation of more stable compounds than initially intended.
Q & A
What is polystyrene and how is it made?
-Polystyrene is a polymer made by joining together lots of styrene monomers. It is commonly used as a packing material for its cushioning properties and is derived from benzene, which is primarily obtained from crude oil.
How does the formation of polystyrene from benzene involve an oxidation reaction?
-The formation of polystyrene from benzene involves a dehydrogenation reaction, which is a type of oxidation reaction. In this process, an ethyl group is attached to the benzene and a couple of hydrogens along with a pair of electrons are removed, forming a carbon-carbon bond.
Why does benzene not react with bromine in the same way as alkenes do?
-Benzene does not react with bromine in the same way as alkenes because it lacks true double bonds due to the delocalization of electrons around its ring. This makes benzene less nucleophilic and requires a catalyst to facilitate the reaction.
What is the role of a catalyst in the electrophilic aromatic substitution reaction of benzene?
-A catalyst, such as ferric bromide (FeBr3) in the case of bromination, helps to polarize the bromine molecule, making it a stronger electrophile. This allows the bromine to react with the benzene ring, leading to electrophilic aromatic substitution.
How does the nitration of benzene differ from the sulfonation of benzene?
-The nitration of benzene involves the replacement of a hydrogen atom on the benzene ring with an NO2 group, using a nitronium ion (N-O-2-plus) as the electrophile. In contrast, sulfonation involves the addition of an S-O-3-H group, using a different electrophile derived from the reaction of sulfur trioxide with concentrated sulfuric acid.
What is the Friedel-Crafts alkylation and how does it contribute to the chemical industry?
-Friedel-Crafts alkylation is a reaction where an alkyl chloride reacts with a Lewis acid, such as aluminum chloride, to attach an alkyl group to a benzene ring. This reaction is significant in the chemical industry as it allows for the creation of various industrially important chemicals, including polymers, dyes, and pharmaceuticals.
What is the difference between Friedel-Crafts alkylation and Friedel-Crafts acylation?
-Friedel-Crafts alkylation involves the addition of an alkyl group to a benzene ring, while Friedel-Crafts acylation involves the addition of an acyl group. Acylation uses an acid chloride and a metal catalyst, such as aluminum chloride, to attach an acetyl group to the benzene ring, forming aromatic ketones like acetophenone.
Why is it important to consider carbocation rearrangement in Friedel-Crafts reactions?
-Carbocation rearrangement is important because more stable carbocations can form, leading to different products than expected. For instance, a reaction with 1-chloro-2-methylpropane may result in tert-butylbenzene instead of 2-methylpropylbenzene due to the greater stability of the tertiary carbocation.
What is the significance of the aromaticity in the electrophilic aromatic substitution reaction?
-Aromaticity is significant because it drives the reaction mechanism to restore the aromatic ring after the initial electrophilic attack. The strong resonance stabilization of the aromatic ring ensures that the reaction proceeds to maintain this stability, even when a catalyst for deprotonation is not explicitly shown.
What are the health and safety concerns associated with nitrobenzene?
-Nitrobenzene is a likely carcinogen that can cause damage to the nervous system, impair vision, and irritate the lungs. It is also used in the production of explosives, making it a hazardous substance that requires careful handling and adherence to safety data sheets.
How does the presence of a catalyst affect the electrophilic aromatic substitution reaction with halogens?
-The presence of a catalyst, such as ferric bromide for bromination, is necessary for halogens to react with benzene. The catalyst helps to polarize the halogen molecule, increasing its electrophilicity and enabling it to react with the benzene ring to form the substituted product.
What is the role of resonance in stabilizing the carbocation intermediate formed during electrophilic aromatic substitution?
-Resonance plays a crucial role in stabilizing the carbocation intermediate by delocalizing the positive charge over the ring. This delocalization reduces the overall energy of the intermediate, making the reaction energetically favorable and allowing it to proceed towards the formation of the substituted aromatic compound.
Outlines
🌟 Introduction to Benzene and Electrophilic Aromatic Substitution
The video begins with an introduction to organic chemistry, specifically focusing on benzene and its unique properties. Deboki Chakravarti explains that benzene, a common component in many everyday substances, is derived from crude oil and contains a ring of carbon atoms with delocalized electrons, making it less reactive than alkenes. The discussion then shifts to how benzene can be chemically modified through electrophilic aromatic substitution, a process that involves the addition of a positively charged species (electrophile) to the benzene ring, resulting in a new compound while maintaining the ring's aromaticity. The video also covers the role of catalysts and the importance of the pi system in these reactions.
🔬 Exploring Electrophilic Aromatic Substitution Reactions
This paragraph delves deeper into the specifics of electrophilic aromatic substitution reactions, including bromination and chlorination, where a hydrogen atom on the benzene ring is replaced by a halogen. The video explains the need for a catalyst, such as ferric bromide, to polarize the bromine molecule and facilitate the reaction. It also touches on nitration, where the benzene ring is substituted with an NO2 group, and the historical significance of this reaction. The paragraph highlights the importance of safety when handling potentially hazardous chemicals like nitrobenzene and emphasizes the use of safety data sheets. The video concludes this section by discussing the synthesis of benzene and its derivatives, including the early work of Eilhardt Mitscherlich.
🔬 Friedel-Crafts Alkylation and Acylation: Carbon-Carbon Bond Formation
The final paragraph introduces the Friedel-Crafts reactions, which are significant for forming carbon-carbon bonds on benzene rings. The video explains two types of reactions: alkylation, where an alkyl group is added to benzene, and acylation, where an acyl group is added. The mechanism for both reactions is described, involving the formation of a carbocation intermediate and the subsequent loss of a proton to restore the aromatic system. Specific examples, such as the production of isopropylbenzene (cumene) from isopropyl chloride and the synthesis of acetophenone from acetyl chloride, are provided. The video also cautions about carbocation rearrangements that can lead to unexpected products and the importance of understanding these reactions for the synthesis of a wide range of industrial chemicals.
Mindmap
Keywords
💡Polystyrene
💡Benzene
💡Electrophilic Aromatic Substitution
💡Catalyst
💡Nitration
💡Friedel-Crafts Alkylation
💡Friedel-Crafts Acylation
💡Carbocation
💡Resonance
💡Isopropylbenzene (Cumene)
💡Acyl Halide
Highlights
Polystyrene is made by joining together lots of styrene monomers.
Benzene rings are present in many everyday substances, making attaching groups to them important for the chemical industry.
Benzene does not react with bromine like alkenes do; a catalyst is required for electrophilic aromatic substitution.
Benzene's electrons are delocalized, resulting in bonds that are like one-and-a-half bonds rather than true double bonds.
Electrophilic aromatic substitution involves swapping a hydrogen on the benzene ring for an electrophilic atom or group.
Ferric bromide (FeBr3) acts as a Lewis acid to polarize bromine, facilitating the electrophilic aromatic substitution with benzene.
Electrophilic aromatic substitution with chlorine is called chlorination, analogous to bromination.
Nitration of benzene involves replacing a hydrogen atom with an NO2 group to form nitrobenzene, which has various industrial uses and health risks.
Nitrobenzene was first prepared in 1834 by Eilhardt Mitscherlich, who also proved benzene's formula and named it.
Sulfonation of benzene involves adding an S-O3H group to the ring using fuming sulfuric acid.
Friedel-Crafts alkylation is a method to add alkyl groups to benzene rings using alkyl halides and a Lewis acid like aluminum chloride.
Friedel-Crafts acylation involves adding an acyl group to benzene using an acid chloride and a metal catalyst.
The drive for the benzene ring to become aromatic again is very strong, often not requiring explicit deprotonation by a base in the mechanism.
In Friedel-Crafts alkylation, carbocations can rearrange to form more stable carbocations before reacting with benzene.
Friedel-Crafts reactions are fundamental in synthetic organic chemistry for forming carbon-carbon bonds on aromatic rings.
The mechanism of electrophilic aromatic substitution involves the formation of a carbocation intermediate that is stabilized by resonance but not fully aromatic.
Concentrated sulfuric acid and nitric acid react to form the nitronium ion, a powerful electrophile for nitration of benzene.
Friedel-Crafts alkylation with isopropyl chloride produces cumene, which is used to synthesize important chemicals like acetone and phenol.
Transcripts
Browse More Related Video

18.2 Friedel Crafts Alkylation and Acylation | Organic Chemistry

More EAS & Benzylic Reactions: Crash Course Organic Chemistry #39

18.1 Electrophilic Aromatic Substitution | Organic Chemistry

Friedel-Crafts Alkylation
Aromatic Halogenation Mechanism - Chlorination, Iodination & Bromination of Benzene

More EAS - Electron Donating and Withdrawing Groups: Crash Course Organic Chemistry #38
5.0 / 5 (0 votes)
Thanks for rating: