Polymer Chemistry: Crash Course Organic Chemistry #35
TLDRCrash Course Organic Chemistry explores the fascinating world of polymers, which are large molecules composed of hundreds to millions of identical subunits called monomers. The video script narrated by Deboki Chakravarti delves into both natural and synthetic polymers, highlighting the accidental discovery of polytetrafluoroethylene (PTFE), better known as Teflon, and its non-stick properties. It explains the formation of polymers through addition polymerization, which involves radicals, cations, or anions, and condensation polymerization, which produces byproducts like water. The episode also discusses the significance of polymer morphology, such as the difference between high-density polyethylene (HDPE) and low-density polyethylene (LDPE), and how it influences a polymer's properties. Furthermore, the script touches on the importance of understanding polymer behavior at various temperatures, as exemplified by the Challenger shuttle disaster. The episode concludes with a teaser for the next topic on benzene and aromatic compounds, emphasizing the relevance of polymer chemistry in our daily lives.
Takeaways
- 🍳 The discovery of polytetrafluoroethylene (PTFE), known as Teflon, was accidental and came from Dr. Roy Plunkett's work with tetrafluoroethylene at DuPont.
- 🧬 Polymers are large molecules made up of hundreds to millions of small, identical subunits called monomers, and they can be both naturally occurring (like DNA and proteins) and synthetic (like plastics).
- 📦 Simple addition polymers, such as polyethylene bags and polystyrene packaging, are formed by repeatedly adding the same monomers together and are named by prefixing 'poly' to the monomer name.
- 🔄 The structure of polymers like polystyrene can be represented using a repeat unit with brackets and an 'n' to indicate multiple monomers in the chain.
- 🤝 Copolymers are addition polymers made from more than one type of monomer, and they are distinguished by using different letters for different repeat units, like in ABS plastic made from acrylonitrile, butadiene, and styrene.
- 🔬 Addition polymerization can occur via three mechanisms: free radical, cationic, and anionic, each using a different type of initiator and process for chain growth and termination.
- ⚙️ Condensation polymers, also known as step-growth polymers, are formed by reactions that release a small molecule, often water, during the joining of monomers.
- 🛡️ Kevlar is an example of a condensation polymer, discovered by Stephanie Kwolek at DuPont, and is known for its exceptional strength, used in applications like bulletproof vests.
- 🥤 Lexan is another condensation polymer, a type of polycarbonate plastic used in food packaging, drink bottles, and even in eyeglass lenses and some cell phone casings.
- 🧊 The properties of a polymer can vary greatly depending on its morphology, which is influenced by its structure, such as being linear or branched, and whether it is crystalline or amorphous.
- 🌡️ The glass transition temperature (Tg) of a polymer determines its behavior at different temperatures, with polymers becoming flexible above Tg and brittle below it, as illustrated by the Challenger shuttle disaster.
- 🔍 Understanding polymer chemistry is crucial as polymers are used in a wide array of applications, from everyday items to advanced materials in medicine and technology.
Q & A
What is the significance of the discovery of polytetrafluoroethylene (PTFE)?
-The discovery of polytetrafluoroethylene (PTFE), also known as Teflon, was significant because it led to the creation of a non-stick coating for cookware. This material was accidentally found by Dr. Roy Plunkett when he discovered a white solid, PTFE, formed from the polymerization of tetrafluoroethylene gas.
What are monomers?
-Monomers are small, identical subunits that make up polymers. They are the building blocks that can be joined together to form larger, more complex molecules known as polymers.
How are simple addition polymers named?
-Simple addition polymers are named by placing the prefix 'poly' before the name of the monomer. For example, polystyrene is made up of thousands of styrene monomers joined together.
What is the abbreviation method used to represent the structure of a polymer?
-The abbreviation method uses brackets around the molecular motif that repeats through the polymer structure, known as the repeat unit. The number 'n' to the bottom right of the brackets indicates that there are many repeats of the unit along the polymer chain.
What is a copolymer?
-A copolymer is an addition polymer that is formed from more than one type of monomer. Different letters are used to distinguish different repeat units in the polymer chain.
How does free radical polymerization initiate?
-Free radical polymerization initiates with a radical, which is a molecule with an unpaired electron. This radical, often generated from an organic peroxide, starts the reaction by forming a bond with one of the carbon atoms in the monomer's double bond, creating a new radical that can then add to another monomer.
What is the process called when two free radicals collide and produce a final molecule in the polymerization process?
-The process is called termination. It is a random event that occurs at different points for different chains, resulting in polymer chains of varying lengths.
How does cationic polymerization differ from free radical polymerization?
-Cationic polymerization uses a positively charged cation as an initiator, which transfers a positive charge to the monomer. Unlike free radical polymerization, where two growing chains colliding can cause termination, in cationic polymerization, they undergo chain transfer reactions instead.
What is the role of water in the anionic polymerization of cyanoacrylates?
-In anionic polymerization of cyanoacrylates, water acts as an initiator. The water molecule attacks the double bond in the monomer, forming an anion that can then react with another monomer, lengthening the polymer chain.
What is Kevlar and how was it discovered?
-Kevlar is a condensation polymer known for its high strength, used in applications such as bulletproof vests and fighter plane panels. It was discovered by Stephanie Kwolek at DuPont in 1965 when she found that a sample of a polymer mix she was working with had formed a strong, thin, and cloudy solution.
What is the difference between high density polyethylene (HDPE) and low density polyethylene (LDPE)?
-HDPE is very linear with few branches, allowing the polymer molecules to pack closely together, resulting in a harder, less flexible plastic. LDPE, on the other hand, has a highly branched structure which prevents tight packing and results in a softer, more flexible plastic.
What is the glass transition temperature (Tg) of a polymer?
-The glass transition temperature (Tg) is the temperature at which a polymer transitions from a hard, glassy state to a rubbery, soft, and flexible state upon heating.
How did polymer chemistry contribute to the Challenger shuttle disaster?
-The Challenger shuttle disaster was partly caused by the failure of O-rings, which are polymer seals. The temperature at the launchpad dropped below the glass transition temperature (Tg) of the polymer used in the O-rings, causing them to become brittle and lose elasticity, leading to a fuel leak and the subsequent explosion.
Outlines
🧪 Introduction to Polymers and Their Discovery
The first paragraph introduces the topic of polymers, highlighting the accidental discovery of polytetrafluoroethylene (PTFE), commonly known as Teflon, by Dr. Roy Plunkett at DuPont. It explains how PTFE, formed from the polymerization of tetrafluoroethylene, became a non-stick coating for cookware. The paragraph also delves into the structure of polymers, which are large molecules composed of many identical subunits called monomers. It distinguishes between natural polymers like DNA, RNA, and proteins, and synthetic polymers like plastics. The formation of addition polymers through a process that involves the repeated addition of monomers is also covered, with examples including polystyrene and polyethylene. The paragraph concludes with a mention of copolymers, which are made from more than one type of monomer, and the use of abbreviations to represent the structure of polymers.
🔬 Polymerization Reactions and Their Types
The second paragraph explores the different types of polymerization reactions used to form addition polymers: free radical, cationic, and anionic polymerization. It describes the initiation, propagation, and termination steps in free radical polymerization, using polyethylene as an example. The paragraph then explains cationic polymerization, where positively charged ions initiate the reaction, and anionic polymerization, which involves negatively charged ions and is exemplified by the creation of superglue. The text also touches on condensation polymers, which are formed by reactions that release small molecules, often water. Kevlar and Lexan are given as examples of condensation polymers, with a brief explanation of their synthesis and properties. The paragraph concludes with a discussion on the commercial synthesis of condensation polymers, mentioning the use of phosgene and alternative, less toxic methods.
🌡️ Polymer Morphology and Temperature Effects
The third paragraph discusses how the morphology of polymers—their structural arrangement—impacts their properties. It differentiates between high density polyethylene (HDPE) and low density polyethylene (LDPE), explaining how their structural differences lead to variations in flexibility and hardness. The concept of polymer structures being either crystalline or amorphous is introduced, using the analogy of uncooked and cooked spaghetti to illustrate the difference. The paragraph also explains how polymers can have both crystalline and amorphous regions, which contribute to a balance of strength and flexibility. The glass transition temperature (Tg) is defined as the point at which a polymer becomes flexible, and it is noted that exceeding Tg can cause the polymer to melt. The consequences of polymer behavior at different temperatures are highlighted through the example of the Challenger shuttle disaster, which was caused by the failure of O rings due to a temperature drop below their Tg. The paragraph concludes by emphasizing the importance of understanding polymer chemistry due to the widespread use of organic polymers.
Mindmap
Keywords
💡Polymers
💡Monomer
💡Polytetrafluoroethylene (PTFE)
💡Addition Polymerization
💡Condensation Polymerization
💡Molecular Morphology
💡Crystalline vs. Amorphous
💡Glasss Transition Temperature (Tg)
💡Copolymers
💡Free Radicals
💡Ions in Polymerization
Highlights
The creation of non-stick coating, Teflon, was discovered accidentally by Dr. Roy Plunkett at DuPont when he found polytetrafluoroethylene (PTFE) had formed from tetrafluoroethylene.
Polymers are large molecules composed of hundreds to millions of small, identical subunits known as monomers.
Biological polymers like DNA, RNA, and proteins play a significant role in organic chemistry.
Human-made polymers such as plastics are addition polymers formed by repeatedly adding the same monomers.
The structure of polymers like polystyrene can be simplified using a repeat unit and the notation 'n' to represent multiple monomers.
Copolymers are formed from more than one type of monomer, as seen in the ABS copolymer used in Lego bricks.
Addition polymerization can occur via free radical, cationic, or anionic mechanisms, each with a distinct initiation and termination process.
Kevlar, a condensation polymer, was discovered by Stephanie Kwolek at DuPont and is now used in bulletproof vests and fighter plane panels.
Condensation polymers, unlike addition polymers, produce a small molecule, often water, as a byproduct when monomers join.
Polymer morphology, the study of different structural arrangements of the same polymer, influences its properties.
High density polyethylene (HDPE) is linear and closely packed, resulting in a harder plastic, while low density polyethylene (LDPE) is branched and more flexible.
Polymers can be crystalline, where the structure is orderly, or amorphous, where the structure is disordered.
The degree of crystallinity in a polymer affects its balance of strength, rigidity, and brittleness.
The glass transition temperature (Tg) of a polymer determines its state (rigid or flexible) at different temperatures.
The Challenger shuttle disaster was linked to the Tg of the O rings' polymer, which became brittle due to cold temperatures.
Understanding polymer chemistry is crucial for various applications, including the development of new materials and the analysis of existing ones.
Next episode of Crash Course Organic Chemistry will explore benzene and aromatic compounds, as well as the use of NMR for structural analysis.
Transcripts
Browse More Related Video
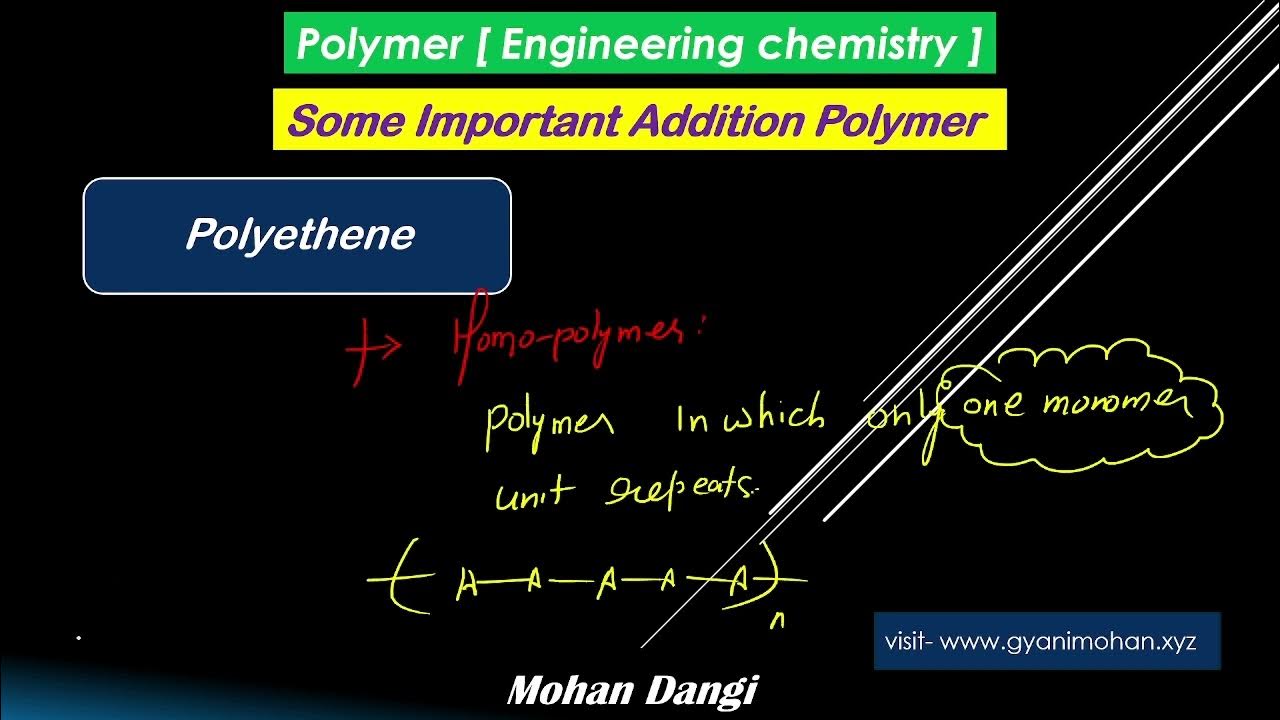
Polymer | polyethene | types of polyethene | engineering chemistry | mohan dangi | RGPV | UPTU
Polymers: Crash Course Chemistry #45

Polymers - Basic Introduction

33. Polymers II (Intro to Solid-State Chemistry)
Condensation Polymerisation | Organic Chemistry | Chemistry | FuseSchool

Uses Of Polymers | Organic Chemistry | Chemistry | FuseSchool
5.0 / 5 (0 votes)
Thanks for rating: