pH and Buffers
TLDRIn this chemistry essentials video, Mr. Andersen discusses the critical role of pH and buffers in maintaining the stability of proteins in our blood. He explains that blood proteins must operate within a narrow pH range of 7.35 to 7.45, and deviations can lead to denaturation. To prevent this, the body employs a buffering system involving carbonic acid, a weak acid, and its conjugate base. This system allows the blood to resist pH changes by shifting the equilibrium in response to added acids or bases. The video also explores the concept of pKa, which is the equilibrium constant for the reversible reaction, and how keeping the pKa value close to the pH value contributes to a stable pH. Furthermore, Mr. Andersen explains the importance of having equal concentrations of the weak acid and its conjugate base for an effective buffer. He concludes with the relevance of pH and pKa in biological systems, such as how changes in pH can affect the behavior of amino acids in proteins, and the use of acid-base indicators to monitor pH changes.
Takeaways
- 🩸 The pH of blood proteins is crucial for their function, specifically within the range of 7.35 to 7.45.
- 🛡️ Buffer solutions help maintain a stable pH by using a weak acid and its conjugate base to counteract added acids or bases.
- ⚖️ The equilibrium of a buffer solution is influenced by the pKa value, which is the equilibrium constant for the reversible reaction.
- 🔄 Le Chatelier's principle explains how a buffer solution resists pH changes by shifting the equilibrium in response to added protons or hydroxide ions.
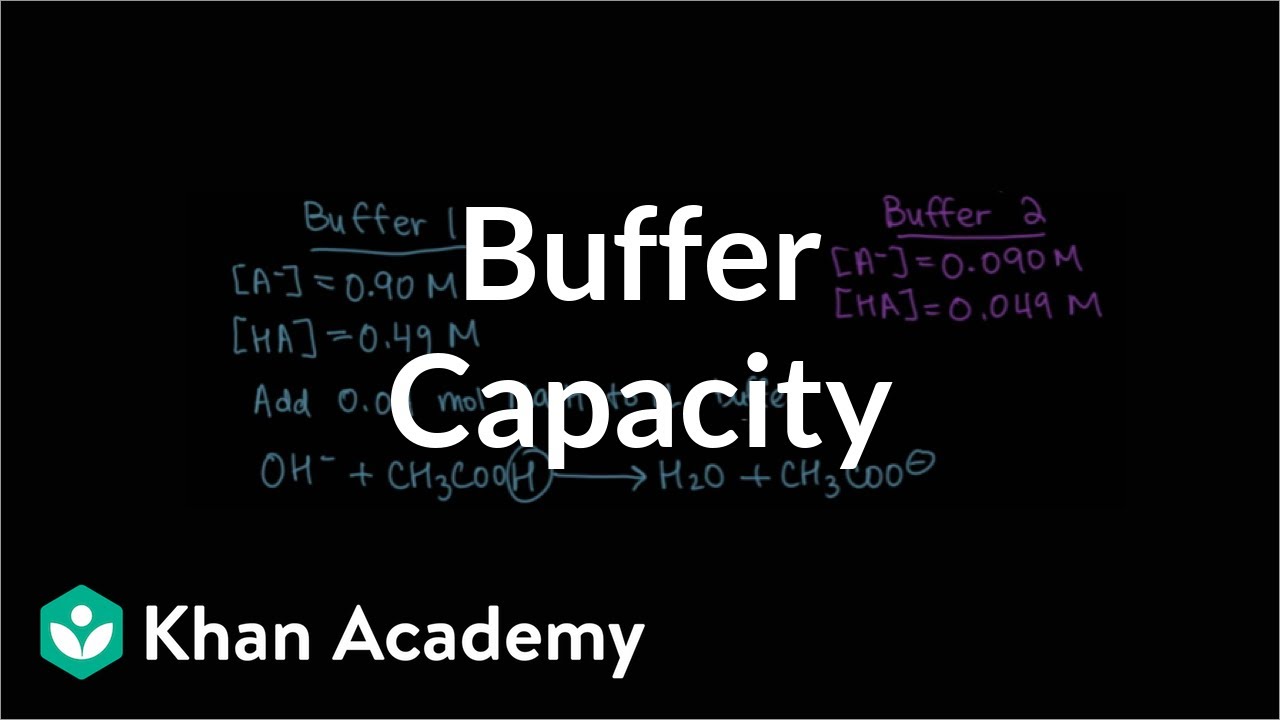
- 📉 To maintain a stable pH, the concentration of the weak acid and its conjugate base should be equal, which increases the buffer capacity.
- 🧪 The equilibrium constant (Ka) for a buffer can be manipulated algebraically to isolate and understand the concentration of hydronium ions.
- 📌 A good buffer solution is achieved when the Ka value or pH is equal to the pKa, resulting in minimal pH changes despite the addition of acids or bases.
- 🔵🟡 The color change in acid-base indicators like bromothymol blue is an example of how pH changes can be visually monitored, with yellow indicating more acid and blue indicating more base.
- 🌟 Biological importance is highlighted by how pH affects protein structure and function, as each amino acid within a protein has a unique side chain with a different pKa value.
- ⚠️ Changes in pH in relation to the pKa value can indicate whether a system is becoming more acidic or basic.
- 🧪 Designing a buffer solution involves balancing a weak acid with its conjugate base to create a system that resists pH changes.
Q & A
What is the pH range that the proteins in our blood must maintain to function properly?
-The proteins in our blood must maintain a pH range between 7.35 and 7.45 to function properly.
What happens if the pH of blood proteins changes radically from the required range?
-If the pH of blood proteins changes radically from the required range, they start to denature and can't carry out their intended functions, such as carrying oxygen and carbon dioxide.
What is a buffer solution and how does it help maintain the pH of blood?
-A buffer solution is a solution that resists changes in pH when small amounts of an acid or a base are added to it. It helps maintain the pH of blood by using a weak acid and its conjugate base to keep the pH stable when protons or hydroxide ions are added.
How does the concentration of a weak acid and its conjugate base affect the buffer capacity?
-The buffer capacity is increased when the concentrations of the weak acid and its conjugate base are kept equal. This allows the buffer to effectively resist changes in pH.
What is the role of pKa in maintaining the stability of pH in a reversible reaction?
-The pKa, which is the equilibrium constant of the reversible reaction, plays a crucial role in maintaining the stability of pH. If the pKa is kept equal to or around the pH, it helps keep the pH stable.
How does LeChatelier's principle apply to buffer solutions when a strong acid or base is added?
-According to LeChatelier's principle, if a strong acid is added, the reaction will shift towards the weak acid side to neutralize the excess protons. Conversely, if a strong base is added, the reaction will shift towards the conjugate base side. In a good buffer solution, these shifts do not significantly change the pH because the weak acid and conjugate base are present in equal concentrations.
What is the relationship between the equilibrium constant (Ka) and the concentration of hydronium ions?
-The equilibrium constant (Ka) is related to the concentration of hydronium ions by the equation Ka = [H3O+][A-]/[HA], where [H3O+] is the concentration of hydronium ions, [A-] is the concentration of the conjugate base, and [HA] is the concentration of the weak acid.
How does the pH scale relate to the concentration of protons in a solution?
-The pH scale is based on the concentration of protons (H+ ions) in a solution. It is defined as the negative logarithm of the proton concentration, pH = -log[H+].
What is the significance of keeping the pH equal to the pKa value in a buffer solution?
-Keeping the pH equal to the pKa value in a buffer solution ensures that the system is at equilibrium, which is necessary for the buffer to effectively resist changes in pH.
How do changes in pH in relation to the pKa value indicate the presence of more acid or base?
-If the pH is less than the pKa, it indicates that there is more of the weak acid present. If the pH is greater than the pKa, it indicates that there is more of the conjugate base present.
What is an example of a biological application of buffer solutions?
-An example of a biological application of buffer solutions is the maintenance of pH in blood, which is crucial for the proper functioning of proteins like myoglobin and hemoglobin.
How can acid-base indicators, such as bromothymol blue, be used to visually monitor changes in pH?
-Acid-base indicators, like bromothymol blue, change color depending on the pH of the solution. In the case of bromothymol blue, it turns yellow in acidic conditions (lower pH) and blue in basic conditions (higher pH), allowing for a visual indication of pH changes.
Outlines
🧪 Understanding pH and Buffers in Chemistry
This paragraph introduces the concept of pH and buffers with a focus on the importance of maintaining a stable pH in our blood for proteins to function correctly. The video explains that the blood's pH must remain between 7.35 and 7.45 to prevent protein denaturation. A buffering system is used to achieve this stability, involving a weak acid (carbonic acid) and its conjugate base. The buffering action is described, where the addition of protons or hydroxide ions to the system results in a shift that maintains pH stability. The paragraph also touches on the role of pKa as an equilibrium constant and how it affects pH, emphasizing the need to keep the pKa value close to the pH for a stable buffer. The summary concludes with an explanation of how the concentrations of the weak acid and its conjugate base can influence buffer capacity, and how LeChatelier's principle applies to buffer solutions when strong acids or bases are added.
📉 pKa, pH, and the Design of Buffer Solutions
The second paragraph delves into the specifics of how to design a good buffer solution. It emphasizes the importance of keeping the pH and pKa values equal for an effective buffer. The paragraph explains that changes in pH in relation to the pKa value can indicate whether the solution is moving towards being more acidic or basic. It also discusses the concept of equal concentrations of a weak acid and its conjugate base, which is crucial for a buffer's ability to resist pH changes. The use of pKa values to understand the concentrations of reactants and products in a reaction is highlighted. The paragraph concludes with applications of pH and pKa, such as acid-base indicators, using bromothymol blue as an example to illustrate how color changes can indicate pH variations. Additionally, the biological significance of pH and pKa is mentioned, particularly in the context of protein structure and function, where changes in pH can alter the behavior of amino acid side chains within proteins like myoglobin.
Mindmap
Keywords
💡pH
💡Buffers
💡Denaturation
💡Carbonic Acid
💡Conjugate Base
💡pKa
💡Le Chatelier's Principle
💡Hydonium Ion
💡Buffer Capacity
💡Acid-Base Indicator
💡Amino Acids
Highlights
The importance of maintaining a specific pH range (7.35 to 7.45) for blood proteins to function properly.
The use of a buffering system to prevent radical pH changes that could denature blood proteins.
The creation of a buffer solution through the weak acid carbonic acid and its conjugate base.
The definition of a buffer solution and its ability to resist pH changes when protons or hydroxide ions are added.
The role of pH as a measure of proton availability and the goal of keeping it stable.
The significance of the pKa value as an equilibrium constant for maintaining pH stability.
The concept of buffer capacity and how equal concentrations of a weak acid and its conjugate base can increase it.
The application of LeChatelier's principle in buffer solutions to predict pH changes upon addition of acids or bases.
The equilibrium equation for buffer solutions and how it relates to the concentration of hydronium ions.
The strategy for creating a good buffer by keeping the Ka value or pH equal to the pKa value.
The impact of ten-fold changes in the concentration of a weak acid and its conjugate base on pH stability.
The use of pKa values to determine the prevalence of a weak acid or its conjugate base at different pH levels.
The application of buffer solutions in acid-base indicators, such as bromothymol blue, to signal pH changes through color changes.
The biological relevance of pH and pKa values in the behavior of amino acid side chains in proteins.
The practical design of a buffer solution by balancing a weak acid and its conjugate base.
The importance of understanding the relationship between pH and pKa for creating effective buffer solutions.
The educational value of the video in explaining the fundamentals of pH, buffers, and their applications.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: