What You Need to Know About Buffers - AP Chem Unit 8, Topics 8-10
TLDRIn this AP Chemistry video, Jeremy Kug concludes Unit 8 by exploring buffer solutions, which are acid-base mixtures that resist pH changes. Buffers are essential in laboratory settings where certain reactions require a stable pH environment. The video explains that a buffer is formed by mixing a weak acid with its conjugate base or a weak base with its conjugate acid. The Henderson-Hasselbalch equation is introduced to calculate the pH of a buffer, with examples provided to illustrate its application. The concept of buffer capacity is also discussed, which refers to a buffer's ability to neutralize added acid or base without significantly altering its pH. Higher concentrations of buffer components increase this capacity. The video concludes with an invitation to the next session, focusing on the applications of thermodynamics.
Takeaways
- 🧪 Buffer solutions are a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid, designed to resist pH changes.
- 🩸 The human blood has a buffer that maintains a pH of approximately 7.4, which is crucial for physiological processes.
- 🚫 A strong acid and a strong base cannot form a buffer; it must be a weak acid or base with its conjugate counterpart.
- ⚖️ Buffers work by reacting with added acids or bases, thus maintaining a stable pH regardless of the addition.
- 🔢 The Henderson-Hasselbalch equation is used to calculate the pH of a buffer: pH = pKa + log([A-]/[HA]).
- 🧮 To solve buffer pH problems, one must know or look up the acid dissociation constant (Ka) or base dissociation constant (Kb).
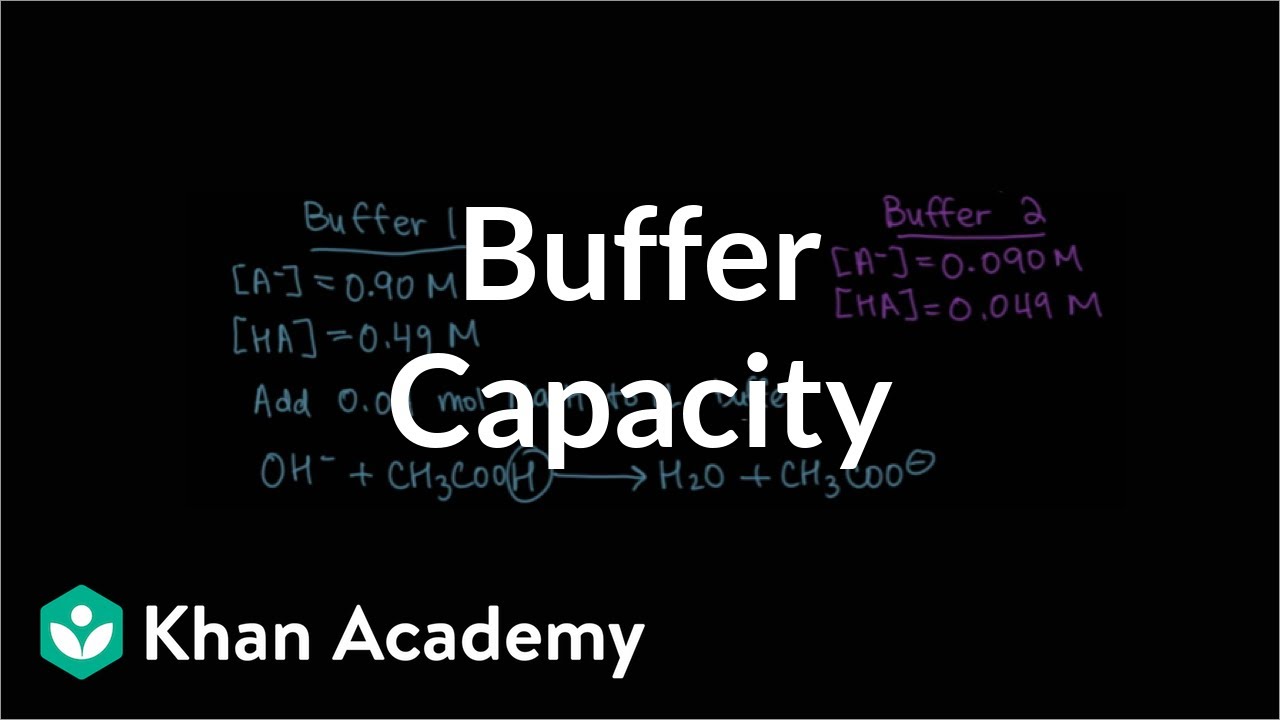
- 📉 A buffer's capacity to neutralize added acid or base without significant pH change is known as its buffer capacity.
- 🔽 More dilute buffer solutions have lower buffer capacity and are less able to withstand the addition of acids or bases.
- 🔼 Higher concentrations of buffer components, while maintaining the correct ratio, increase the buffer's capacity.
- 🤔 The ratio of the conjugate base to the acid in a buffer can be adjusted while keeping the pH the same, but it affects the buffer's capacity.
- ⚖️ A buffer with more conjugate base than acid is more effective at neutralizing added acid, and vice versa for a buffer with more conjugate acid.
- 📚 Understanding buffer solutions, their capacity, and the role of weak acids and bases is essential for controlling pH in chemical reactions.
Q & A
What is the main concept discussed in the video?
-The main concept discussed in the video is buffer solutions, which are special types of acid-base mixtures that resist pH changes.
What is a buffer solution typically composed of?
-A buffer solution is typically composed of a weak acid mixed with its conjugate base or a weak base mixed with its conjugate acid.
Why are buffer solutions important in a laboratory setting?
-Buffer solutions are important in a laboratory setting because they help maintain a consistent pH level, which is necessary for certain chemical reactions to occur effectively.
What is the physiological pH of human blood?
-The physiological pH of human blood is approximately 7.4.
How does a buffer work when you add acid or base to it?
-When you add acid to a buffer, the acid reacts with the base in the buffer, and when you add base to the buffer, it reacts with the acid, thus maintaining the pH close to the original level.
What equation is used to calculate the pH of a buffer?
-The Henderson-Hasselbalch equation is used to calculate the pH of a buffer.
What is the role of the Ka value in the Henderson-Hasselbalch equation?
-The Ka value is the acid dissociation constant, and it is used in the Henderson-Hasselbalch equation to determine the pH of the buffer.
What is buffer capacity and why is it important?
-Buffer capacity is the ability of a buffer to resist changes in pH upon the addition of significant amounts of acid or base. It is important because it determines how much acid or base a buffer can neutralize without a significant pH change.
Why do we not always use very dilute concentrations in buffer solutions?
-While dilute concentrations can maintain the same pH due to the constant ratio of base to acid, they have a lower buffer capacity and are less effective at neutralizing added acid or base.
How does the ratio of the base to the acid in a buffer affect its ability to neutralize added substances?
-If a buffer has more conjugate acid than base, it can absorb more base without significantly changing its pH. Conversely, if there is more conjugate base than acid, it can absorb more acid without significant pH change.
What is the significance of the example given with hydrochloric acid and sodium fluoride in calculating the pH of a buffer?
-The example demonstrates how to use the Henderson-Hasselbalch equation to calculate the pH of a buffer solution containing a specific amount of a weak acid and its conjugate base.
What is the purpose of maintaining a certain pH in a buffer solution?
-The purpose of maintaining a certain pH in a buffer solution is to ensure that certain reactions can take place effectively, as some chemical reactions are sensitive to pH levels.
Outlines
🧪 Understanding Buffer Solutions
Jeremy Kug introduces the concept of buffer solutions in AP Chemistry, focusing on their composition and purpose. A buffer is an acid-base mixture, typically consisting of a weak acid and its conjugate base or a weak base and its conjugate acid. Buffers are used in the lab to maintain a consistent pH level, which is crucial for certain chemical reactions. An example given is the human blood buffer, which keeps the pH around 7.4. The video also discusses how to identify a buffer mixture and the role of the Henderson-Hasselbalch equation in calculating the pH of a buffer.
📐 Calculating pH of a Buffer
The second paragraph delves into the calculation of a buffer's pH using the Henderson-Hasselbalch equation. An example problem is presented where a student must calculate the pH of a buffer solution containing hydrochloric acid and sodium fluoride. The process involves looking up the acid's dissociation constant (KA) and plugging various concentrations into the equation to solve for pH. The video also touches on the concept of buffer capacity, explaining that more concentrated buffer components can withstand larger additions of acid or base without significant pH changes.
🔍 Buffer Capacity and Neutralization
The final paragraph discusses buffer capacity in more detail. It explains that higher concentrations of buffer components, while maintaining the same ratio, increase the buffer's ability to neutralize added acid or base. The video illustrates this by comparing two different concentrations of a buffer with the same base-to-acid ratio. It concludes by emphasizing that a buffer with more conjugate base than acid will be more effective at neutralizing added acid, and vice versa for a buffer with more conjugate acid. The video ends with an invitation to the next unit on the applications of thermodynamics.
Mindmap
Keywords
💡Buffer Solutions
💡Weak Acid
💡Conjugate Base
💡pH
💡Henderson-Hasselbalch Equation
💡pKa
💡Buffer Capacity
💡Neutralization
💡Conjugate Acid
💡Logarithm
💡Scientific Calculator
Highlights
Buffer solutions are a special type of acid-base mixture that resists pH change, essential for maintaining a consistent pH in certain laboratory reactions.
Buffers are created by mixing a weak acid with its conjugate base or a weak base with its conjugate acid.
The human blood has a buffer that maintains its pH at approximately 7.4, which is crucial for physiological processes.
To form a buffer, one needs a weak acid and its conjugate base, such as acetic acid and acetate ion.
Sulfuric acid, being a strong acid, is not suitable for creating an effective buffer.
Buffers work by reacting with added acids or bases, maintaining the pH regardless of the addition.
The Henderson-Hasselbalch equation is used to calculate the pH of a buffer solution.
The pH of a buffer can be determined by knowing the pKa of the weak acid and the concentrations of the acid and its conjugate base.
An example calculation is provided, showing how to determine the pH of a buffer with hydrochloric acid and sodium fluoride.
To create a buffer with a specific pH, one can adjust the ratio of the conjugate base to the weak acid while keeping their concentrations in proportion.
Buffer capacity refers to the ability of a buffer to withstand the addition of acid or base without significant pH change.
Higher concentration buffer components increase the buffer's capacity to neutralize added acid or base.
The ratio of the base to the acid in a buffer determines its effectiveness at neutralizing either added acid or base.
Buffers with more conjugate acid can absorb more base, while buffers with more conjugate base can absorb more acid.
The practical application of buffer solutions is discussed, including their importance in biological systems and laboratory settings.
The video concludes with an encouragement to learn more about buffers and their role in acid-base chemistry.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: