Temperature vs Heat - Explained
TLDRThis educational video delves into the concepts of temperature and heat, clarifying their differences. It explains that temperature is a measure of the average kinetic energy of particles in a substance, with higher temperatures correlating to increased particle movement and kinetic energy. Conversely, heat is the flow of energy due to temperature differences, measured in joules, and always transfers from hotter to colder objects. The video uses simulations to illustrate these scientific principles, emphasizing the direct relationship between temperature and kinetic energy, and the transfer of heat from hot to cold substances until thermal equilibrium is reached.
Takeaways
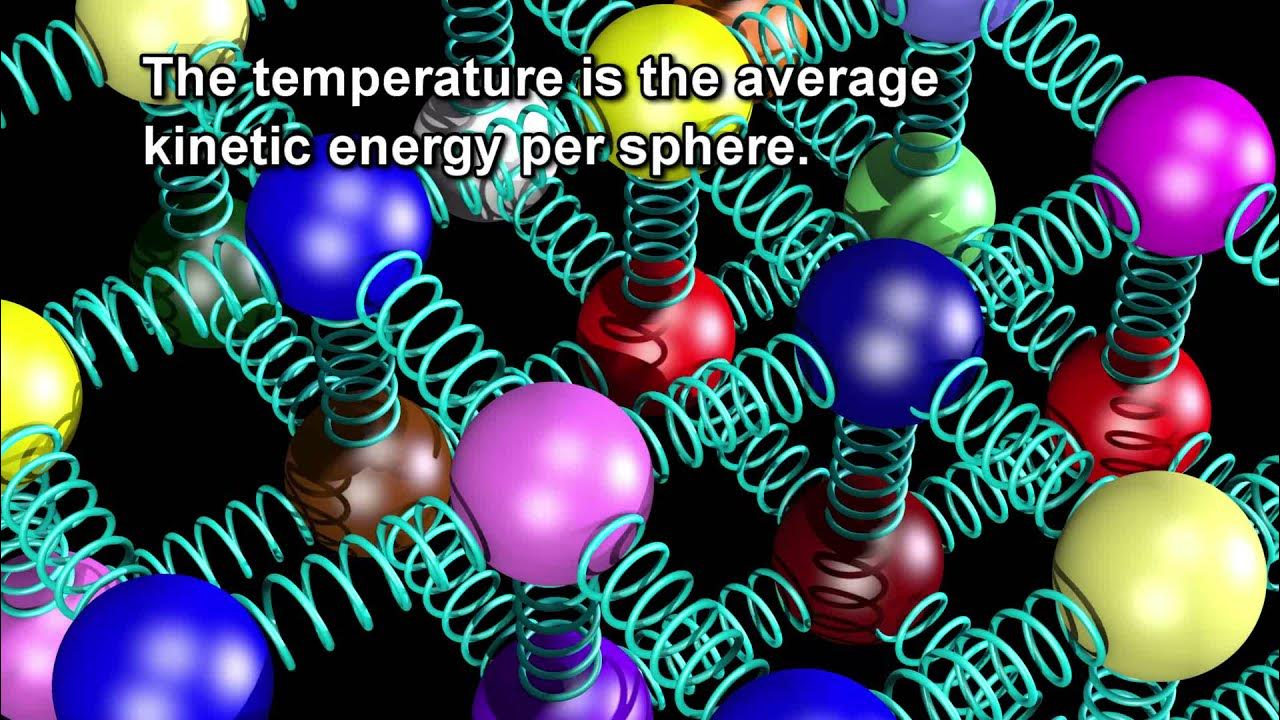
- 🌡️ Temperature is a measure of the average kinetic energy of particles in a substance, indicating how fast they are moving.
- 🔥 Higher temperatures correspond to greater kinetic energy and faster particle movement, while lower temperatures result in slower movement and less kinetic energy.
- 📈 The relationship between temperature and kinetic energy is directly proportional; as one increases, so does the other.
- 🌬️ Gas particles, such as those in a simulation, demonstrate the effect of temperature changes on their motion and kinetic energy.
- ⚙️ Absolute zero (0 K) is the theoretical lowest possible temperature where particle motion stops and kinetic energy is at its minimum.
- 🔄 Heat is distinct from temperature and is defined as the flow of energy due to temperature differences between objects, measurable in joules.
- 🔽 Heat always flows from hotter to colder objects, aiming to equalize temperature differences.
- 🥣 An example with water in Styrofoam containers illustrates heat transfer, where the final temperature is the average of the initial temperatures of the two sides.
- 🧱 A simulation with a hot water and a cold brick shows heat transfer in action, with the brick absorbing heat and the water losing some, until they reach the same temperature.
- 🌡️🔄 The use of a thermometer in the simulation helps visualize how temperature changes as a result of heat transfer between objects.
- 📚 The University of Colorado PhET simulations provide interactive and visual ways to understand concepts of temperature, heat, and energy transfer.
Q & A
What is the definition of temperature?
-Temperature is a measure of the average kinetic energy in the particles of a substance. It indicates how fast the particles in a substance are moving.
How does the temperature affect the kinetic energy of particles?
-As the temperature increases, the kinetic energy of the particles also increases, causing them to move faster. Conversely, when the temperature decreases, the kinetic energy of the particles decreases, and they slow down.
What happens to gas particles when the temperature of the gas is increased?
-When the temperature of a gas is increased, the gas particles start to move faster, thereby gaining more kinetic energy.
What is the relationship between temperature and kinetic energy?
-The relationship between temperature and kinetic energy is directly proportional. As the temperature rises, the kinetic energy of the particles increases, and as the temperature falls, the kinetic energy decreases.
What is the definition of heat?
-Heat is a measure of the flow of energy due to temperature differences between objects. It always flows from hot to cold and can be measured in joules.
How does heat transfer occur between objects?
-Heat transfer occurs between objects when there is a temperature difference. Heat flows from the hotter object to the colder one until both objects reach the same temperature, at which point the heat transfer stops.
What happens when an object is cooled down to absolute zero?
-When an object is cooled down to absolute zero (0 K), the particles stop moving, and their kinetic energy becomes zero. This is the coldest possible temperature in the known universe.
How can the final temperature be determined when two substances with different temperatures are combined?
-The final temperature when two substances are combined can be determined by taking the average of the initial temperatures of the substances, assuming equal masses and no additional heat loss or gain.
What is the role of the Styrofoam container with a thin metal piece in the example?
-The Styrofoam container with a thin metal piece is used to demonstrate heat transfer between two substances with different temperatures. The metal piece conducts heat, causing the temperatures of the two substances to equalize over time.
How does the kinetic energy of particles change when heat is applied?
-When heat is applied to particles, their kinetic energy increases. This results in the particles moving faster and with more force as their velocity increases.
What can the Phet simulation from the University of Colorado demonstrate?
-The Phet simulation from the University of Colorado can demonstrate various scenarios of heat transfer, showing how temperature differences lead to the flow of energy (heat) between objects and how this affects the kinetic energy of particles.
Outlines
🌡️ Understanding Temperature and Kinetic Energy
This paragraph introduces the concept of temperature as a measure of the average kinetic energy of particles in a substance. It explains that higher temperatures correspond to faster movement and greater kinetic energy of particles, while lower temperatures result in slower movement and less kinetic energy. The relationship between temperature and kinetic energy is directly proportional. The paragraph also describes a simulation using the University of Colorado Phet simulations to illustrate how gas particles respond to changes in temperature, highlighting that at absolute zero, particle motion stops, and kinetic energy is at its minimum.
🔥 Heat Transfer and Energy Flow
This section distinguishes between temperature and heat, clarifying that heat is the flow of energy due to temperature differences between objects, measured in joules. It uses a hypothetical example of a Styrofoam container with equal masses of hot and cold water separated by a metal piece to demonstrate heat transfer. The heat flows from the hotter water to the colder, resulting in both reaching an equilibrium temperature of 50 degrees Celsius. The paragraph emphasizes that heat always moves from hot to cold and can be quantified in joules, providing a practical understanding of heat as energy transfer.
🌞 Further Exploration of Heat Transfer
The paragraph further explores the concept of heat transfer through a simulation involving a hot water container and an iron block. It explains that placing a cold iron block in hot water results in the water transferring its thermal energy to the block, causing the water temperature to decrease and the block's temperature to increase until they reach the same level. Another example is given where a thermometer is used to show the temperature changes during heat transfer. The paragraph reinforces the idea that heat is the flow of thermal energy from warmer to cooler substances until thermal equilibrium is achieved.
Mindmap
Keywords
💡Temperature
💡Kinetic Energy
💡Heat
💡Average Kinetic Energy
💡Absolute Zero
💡Joules
💡Heat Transfer
💡Thermal Energy
💡Simulation
💡Temperature Gradient
Highlights
Temperature is a measure of the average kinetic energy in the particles of a substance.
At higher temperatures, particles in a substance have more kinetic energy and move faster.
As temperature decreases, particles slow down and have less kinetic energy.
Kinetic energy is directly proportional to the temperature of the particles.
At absolute zero (0 K), particle motion stops and kinetic energy is at its minimum.
Heat is different from temperature; it is a measure of the flow of energy due to temperature differences.
Heat always flows from hot to cold objects.
Heat can be measured in joules.
When two objects with different temperatures come into contact, they reach an equilibrium temperature.
The University of Colorado Phet simulations provide interactive learning about temperature and heat concepts.
In a simulation, heating a substance increases the kinetic energy of its particles.
Cooling a substance decreases the kinetic energy of its particles.
Heat transfer results in both substances reaching the same temperature over time.
The flow of heat can be visualized and understood through Phet simulations.
Understanding the difference between temperature and heat is crucial for grasping thermal energy concepts.
The relationship between temperature and kinetic energy is fundamental in various scientific fields and applications.
The video provides a comprehensive explanation of the concepts of temperature and heat through both theory and simulation.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: