7 E1 vs E2
TLDRThis lesson delves into the intricacies of E1 and E2 reactions, highlighting the differences in substrate requirements, base strengths, and stereochemical considerations. It emphasizes the importance of Zaitsev's rule in E2 reactions and the preference for more substituted alkenes, while E1 reactions follow a different path, often involving carbocation rearrangements. The video also discusses the impact of leaving groups, solvent types, and the role of bulky bases in determining the major elimination products, providing a comprehensive understanding of these organic chemistry concepts.
Takeaways
- 📚 The stability of alkenes is influenced by substitution; more substitution leads to higher stability and lower energy.
- 📌 Zaitsev's Rule states that the alkene formed with the most substitution (fewest hydrogens) will be the major product in elimination reactions.
- 🔄 Anti-periplanar conformation is a stereochemical requirement for E2 reactions, where the hydrogen and the leaving group must be nearly 180 degrees apart.
- 🌟 In E2 reactions, a strong base is required and the reaction rate is first order with respect to both the base and the substrate, making it second order overall.
- 🔄 Carbocation rearrangements are not a factor in E2 reactions as no carbocation intermediate is formed, unlike in E1 reactions.
- 📌 In E1 reactions, the more substituted the alkyl halide, the faster the reaction, following Zaitsev's Rule.
- 🌟 E1 reactions involve weak bases such as water or alcohols, and the reaction rate is first order with respect to the substrate only.
- 🔄 For E2 reactions, the stereochemistry of the substrate is crucial due to the anti-periplanar requirement, while E1 reactions do not have this concern.
- 🌟 Bulky bases can lead to Hofmann elimination (forming the least substituted alkene) instead of Zaitsev elimination in E2 reactions.
- 📌 Bad leaving groups, such as fluorine, can also lead to Hofmann elimination as the major product in E2 reactions due to slower leaving group departure.
- 🔄 E1 and E2 reactions often compete with each other and can lead to mixtures of products, which is generally undesirable in synthetic chemistry.
Q & A
What is the main difference between E1 and E2 reactions in terms of the number of reactant molecules involved in the rate-determining step?
-In E1 reactions, there is only one reactant molecule involved in the rate-determining step, whereas in E2 reactions, there are two reactant molecules involved in this step.
What is Zaitsev's Rule in the context of elimination reactions?
-Zaitsev's Rule states that the elimination reaction will preferentially form the alkene with the most substituted double bond, which is the more stable alkene due to hyperconjugation.
What is the stereochemical requirement for E2 reactions?
-The stereochemical requirement for E2 reactions is that the beta hydrogen (which is being removed) and the leaving group must be in an anti-periplanar orientation, meaning they are approximately 180 degrees apart.
What is the major product formed when a bulky base is used in an elimination reaction?
-When a bulky base is used, the major product is typically the Hofmann product, which is the less substituted alkene. This is due to the bulky base preferentially deprotonating the primary carbon instead of the more substituted secondary carbons.
What is the effect of a bad leaving group on the elimination reaction?
-A bad leaving group, such as fluorine, can lead to the formation of the Hofmann product as the major product because the reaction slows down and preferentially forms the less substituted alkene due to the buildup of negative charge on the beta carbon in the transition state.
How does the stability of an alkene affect the rate of elimination reactions?
-The more substituted the alkene that is formed, the more stable it is, and thus the faster the elimination reaction will proceed. This is because more substituted alkenes benefit more from hyperconjugation, which stabilizes the molecule.
What is the role of the solvent in E2 reactions?
-While the solvent is not involved in the rate-determining step of E2 reactions, polar protic solvents can slightly increase the rate of reaction. However, E2 reactions can proceed in non-polar solvents as well, although they may be slightly slower.
What is the primary factor to consider when distinguishing between E1 and E2 reactions?
-The primary factor to consider is the strength of the base used. Strong bases favor E2 reactions, while weak bases favor E1 reactions. This is because the presence of a strong base can lead to a concerted elimination mechanism, whereas weak bases tend to lead to a multi-step mechanism involving carbocation formation.
Can you give an example of a bulky base used in elimination reactions?
-Potassium t-butoxide is a common example of a bulky base used in elimination reactions. Its bulkiness leads to preferential deprotonation of the primary carbon, resulting in the formation of the Hofmann product.
How does the presence of a carbocation rearrangement affect the outcome of E1 reactions?
-Carbocation rearrangements can lead to the formation of a more stable carbocation intermediate, which can then lead to the formation of a different alkene product. This can result in a mixture of products and is a key factor to consider when predicting the outcome of E1 reactions.
Outlines
📚 Introduction to Elimination Reactions
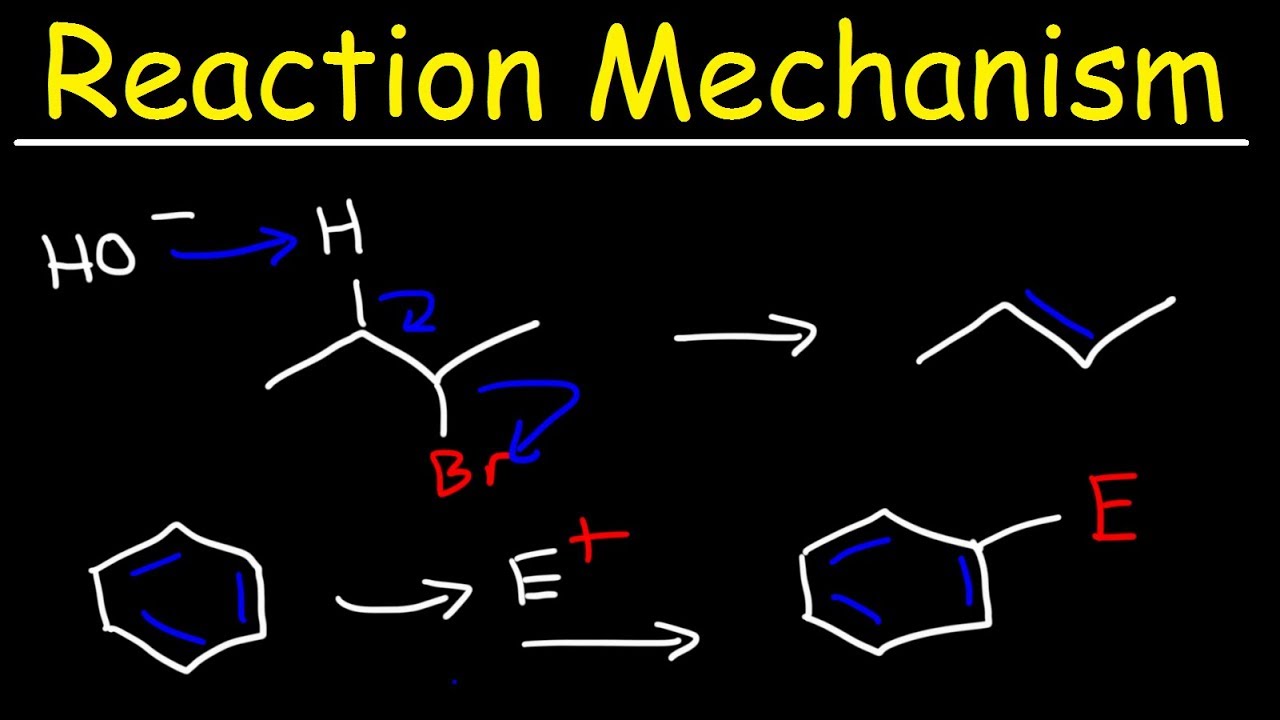
This paragraph introduces the topic of elimination reactions, specifically focusing on the differences between E1 and E2 reactions. It explains the concept of Zaitsev's rule and how it predicts the major product of an elimination reaction based on the stability of the resulting alkene. The importance of the stereochemical requirement for E2 reactions, known as anti-periplanar geometry, is also highlighted. Additionally, the paragraph mentions the role of the base in E2 reactions and the significance of the alkene's substitution pattern on its stability and reactivity.
🧪 Stereochemical Requirements and Zaitsev's Rule
This paragraph delves deeper into the stereochemical requirements for E2 reactions, emphasizing the need for the beta hydrogen and the leaving group to be anti-periplanar. It explains how the number of hydrogens on the beta carbon influences the outcome of the reaction according to Zaitsev's rule, which favors the formation of the more substituted, and thus more stable, alkene. The paragraph also discusses the exceptions to this rule, such as when a bulky base or a poor leaving group leads to the formation of the less substituted alkene, known as Hofmann elimination.
🔄 Understanding Carbocation Stability
The paragraph discusses the stability of carbocations, which are key intermediates in E1 reactions. It explains how the degree of substitution on the carbon atom bearing the positive charge affects the stability of the carbocation, with tertiary carbocations being more stable than secondary or primary carbocations. The paragraph also hints at the possibility of rearrangements in E1 reactions, which can lead to the formation of different alkene products.
🌡️ E1 Reactions: Unimolecular Elimination
This paragraph introduces E1 reactions, describing them as a two-step process involving the formation of a carbocation intermediate. It contrasts E1 with E2 reactions, noting that E1 reactions are favored by weak bases and occur more readily with tertiary and secondary substrates. The paragraph also touches on the rate law for E1 reactions, which is first order with respect to the substrate, and the lack of a requirement for anti-periplanar geometry due to the stepwise mechanism of the reaction.
🔄 Carbocation Rearrangements in E1 Reactions
The paragraph discusses the possibility of carbocation rearrangements in E1 reactions, which can lead to the formation of different alkene products. It explains that while primary substrates do not typically undergo E1 reactions, secondary and tertiary substrates can, and that the more substituted the substrate, the faster the E1 reaction will proceed. The paragraph also notes that unlike E2 reactions, E1 reactions are not subject to the stereochemical requirement of anti-periplanar geometry.
🌟 Comparing E1 and E2 Reactions
This paragraph compares E1 and E2 reactions, highlighting the key differences between them. It notes that E1 reactions involve a carbocation intermediate and are favored by weak bases, while E2 reactions are concerted and require a strong base. The paragraph also emphasizes that E2 reactions are subject to Zaitsev's rule and the anti-periplanar requirement, whereas E1 reactions follow the substrate's reactivity trend based on the stability of the resulting carbocation. Additionally, it mentions that E1 and E2 reactions often compete with each other, leading to mixtures of products.
📈 Predicting Elimination Products
The paragraph focuses on how to predict the major product of elimination reactions, providing examples to illustrate the application of the rules discussed earlier. It shows how to identify the substrate, leaving group, and base, and how these factors determine whether an E1 or E2 reaction will occur. The paragraph also explains how to draw the potential carbocations for E1 reactions and how to predict the major alkene product based on the stability of the resulting alkene.
🎓 Conclusion and Study Tips
In this final paragraph, the speaker wraps up the lesson on elimination reactions by summarizing the key points and providing study tips. They emphasize the importance of understanding the differences between E1 and E2 reactions, the factors that influence their reactivity, and how to predict the major products. The speaker also encourages students to practice and review the material, and offers resources for further study, including subscribing to the channel for more lessons and visiting Chad's Prep for additional videos.
Mindmap
Keywords
💡Elimination Reactions
💡Zaitsev's Rule
💡Hofmann Elimination
💡Stereochemical Requirements
💡Carbocation
💡Substitution Reactions
💡Base
💡Leaving Group
💡Alkenes
💡Hyperconjugation
Highlights
The lesson covers the differences between E1 and E2 reactions, including their mechanisms and factors affecting their rates.
Zaitsev's rule is a key principle in predicting the major product of elimination reactions, stating that the more substituted alkene is formed preferentially.
Hofmann elimination or anti-Zaitsev elimination occurs when the least substituted alkene is formed, often due to the use of a bulky base or a poor leaving group.
E2 reactions require an anti-periplanar conformation for the beta hydrogen and the leaving group, which means they must be approximately 180 degrees apart.
The stereochemical requirement for E2 reactions is crucial for understanding the formation of cis and trans isomers in the products.
E1 reactions involve the formation of a carbocation intermediate, which can lead to rearrangements and different product outcomes compared to E2 reactions.
In E1 reactions, the substrate's level of substitution significantly impacts the reaction rate, with more substituted alkyl halides reacting faster.
E2 reactions are favored in polar aprotic solvents, while E1 reactions are not particularly sensitive to the solvent type.
Strong bases are necessary for E2 reactions, while weak bases are used for E1 reactions, reflecting the different requirements for nucleophilicity and basicity.
E1 and E2 reactions often compete with each other, leading to mixtures of substitution and elimination products.
The lesson provides a comprehensive overview of the factors that influence the direction and outcome of elimination reactions, including substrate structure, base strength, and leaving group ability.
The stability of the formed alkene is a critical factor in determining the preference for either Zaitsev or Hofmann elimination pathways.
The lesson explains the concept of kinetic and thermodynamic products, and how they relate to the major and minor products observed in elimination reactions.
The role of hyperconjugation in stabilizing alkenes is discussed, highlighting the importance of substitution for alkene stability.
The lesson emphasizes the importance of stereochemistry in E2 reactions, with specific examples demonstrating how the spatial arrangement of atoms affects reaction outcomes.
The impact of leaving group ability on the preference for Zaitsev versus Hofmann elimination is explored, with examples illustrating how a poor leaving group can lead to the formation of the less substituted alkene.
Transcripts
Browse More Related Video

7.6 E1 Reactions and E1 vs E2 | Organic Chemistry

SN1 SN2 E1 E2 Reaction Mechanism - Test Review

7.5 E2 Reactions | Organic Chemistry
Organic Chemistry - Reaction Mechanisms - Addition, Elimination, Substitution, & Rearrangement

Organic Chemistry Elimination Reactions - E1, E2, E1CB

12.7 Elimination Reactions of Alcohols | Organic Chemistry
5.0 / 5 (0 votes)
Thanks for rating: