Stoichiometry example problem 2 | Chemistry | Khan Academy
TLDRThe video script explains a stoichiometry problem involving the reaction of glucose with oxygen to produce carbon dioxide and water. It details the process of balancing the chemical equation, calculating the moles of glucose from its atomic weight, and determining the required mass of oxygen and the masses of carbon dioxide and water produced. The conclusion is that 25 grams of glucose requires 26.7 grams of oxygen, and yields 36.7 grams of carbon dioxide and 15 grams of water.
Takeaways
- 🔬 The script is focused on a stoichiometry problem involving the reaction of glucose (C6H12O6) with oxygen to produce carbon dioxide and water.
- 📝 The first step in solving the problem is to balance the chemical equation, emphasizing the importance of this process in stoichiometry.
- 🧮 The calculation involves determining the mass of oxygen required to react with a given mass of glucose, and the masses of carbon dioxide and water produced.
- 🔎 It showcases the method to calculate the molar mass of glucose using atomic weights of carbon (12.011), hydrogen (1.0079), and oxygen (15.999).
- ✏️ The atomic weights are simplified to 12 (carbon), 1 (hydrogen), and 16 (oxygen) for ease of calculation, leading to a molar mass of glucose as 180 g/mol.
- ⚖️ The script demonstrates converting grams of glucose into moles, an essential step in stoichiometry for comparing quantities of reactants and products.
- 🧪 It illustrates the mole-to-mole relationship between reactants and products, highlighting the 1:6 ratio of glucose to oxygen in the reaction.
- 📊 The calculation of oxygen required is shown, converting moles of oxygen into grams, using the molar mass of molecular oxygen (O2) as 32 g/mol.
- 💧 The process includes finding the number of moles and then the mass of carbon dioxide and water produced, with emphasis on different molar masses for these compounds.
- 📌 The final result indicates that 25 grams of glucose requires 26.7 grams of oxygen, producing 36.7 grams of carbon dioxide and 15 grams of water.
Q & A
What is the balanced chemical equation for the reaction between glucose and oxygen?
-The balanced chemical equation is C6H12O6 + 6O2 -> 6CO2 + 6H2O.
How many grams of oxygen are required to react completely with 25 grams of glucose?
-26.7 grams of oxygen are required to react completely with 25 grams of glucose.
What are the products of the reaction between glucose and oxygen?
-The products of the reaction are carbon dioxide (CO2) and water (H2O).
How is the stoichiometry of a chemical reaction used to determine the mass of reactants and products?
-Stoichiometry uses the balanced chemical equation to relate the amounts of reactants and products in moles, which can then be converted to grams using their molar masses.
What is the importance of balancing a chemical equation before solving a stoichiometry problem?
-Balancing a chemical equation ensures the law of conservation of mass is met, providing the correct mole ratio between reactants and products needed for stoichiometric calculations.
What is the molar mass of glucose, and how is it calculated?
-The molar mass of glucose is 180 grams per mole, calculated by summing the molar masses of its constituent elements: 6 carbons (12 g/mol each), 12 hydrogens (1 g/mol each), and 6 oxygens (16 g/mol each).
How many grams of carbon dioxide are produced from the complete reaction of 25 grams of glucose?
-36.7 grams of carbon dioxide are produced from the complete reaction.
How many grams of water are produced from the reaction?
-15.0 grams of water are produced from the reaction.
What is the mole ratio of glucose to oxygen in the balanced equation for their reaction?
-The mole ratio of glucose (C6H12O6) to oxygen (O2) in the balanced equation is 1:6.
Why are atomic weights rounded to simpler numbers in stoichiometry problems?
-Atomic weights are rounded to simpler numbers to simplify calculations, especially in educational or illustrative contexts, while maintaining reasonable accuracy.
Outlines
🧪 Stoichiometry Problem: Glucose Reaction
The paragraph introduces a stoichiometry problem involving the reaction of glucose with oxygen to produce carbon dioxide and water. It presents the challenge of calculating the mass of oxygen needed to react with 25 grams of glucose, as well as the masses of carbon dioxide and water formed. The paragraph begins by writing the chemical reaction and emphasizes the importance of balancing the equation. It then proceeds to balance the equation by adjusting the coefficients, starting with the more complex molecule (glucose) and moving to the simpler ones (oxygen and water).
📈 Calculating Moles and Atomic Weights
This paragraph focuses on calculating the number of moles of glucose from the given mass (25 grams) and determining the atomic weights of carbon, hydrogen, and oxygen. It explains the process of converting grams to moles using the atomic weight of glucose (180 g/mol). The paragraph also discusses the importance of memorizing common atomic weights for solving stoichiometry problems and gives credit to the source of the periodic table used. The calculation leads to determining that there are 0.139 moles of glucose in 25 grams.
🌡️ Determining Moles and Mass of Oxygen
The paragraph continues the stoichiometry problem by calculating the moles of oxygen required for the reaction based on the moles of glucose. It uses the stoichiometric ratios from the balanced equation (1 mole of glucose requires 6 moles of oxygen) to find that 0.833 moles of oxygen are needed. The paragraph then converts these moles of oxygen into grams, using the atomic weight of molecular oxygen (O2, 32 g/mol), resulting in a requirement of 26.7 grams of oxygen.
🌿 Production of Carbon Dioxide and Water
The final paragraph concludes the stoichiometry problem by calculating the masses of carbon dioxide and water produced from the reaction. Using the stoichiometric ratios from the balanced equation, it determines that 0.833 moles of both carbon dioxide and water are produced. The paragraph then calculates the mass of carbon dioxide (36.7 grams) and water (15.0 grams) using their respective atomic weights (44 g/mol for CO2 and 18 g/mol for H2O). The problem's solution is summarized, stating that 25 grams of glucose will require 26.7 grams of oxygen, produce 36.7 grams of carbon dioxide, and 15 grams of water.
Mindmap
Keywords
💡Glucose
💡Oxygen
💡Stoichiometry
💡Carbon Dioxide
💡Water
💡Atomic Weight
💡Molar Mass
💡Balanced Chemical Equation
💡Moles
💡Avogadro's Number
💡Chemical Reaction
Highlights
Introduction to the stoichiometry problem involving glucose reaction with oxygen to produce carbon dioxide and water.
Explanation of the importance of balancing the chemical equation before proceeding with stoichiometry calculations.
Balancing the chemical equation step-by-step, starting with carbon, then hydrogen, and finally oxygen.
Derivation of the atomic weights for carbon, hydrogen, and oxygen from a periodic table.
Simplification of atomic weights to make calculations easier, acknowledging the approximation.
Calculation of the molar mass of glucose (C6H12O6) as 180 grams per mole.
Conversion of 25 grams of glucose to moles using its molar mass.
Use of the balanced chemical equation to determine the mole ratio of glucose to oxygen required for the reaction.
Calculation of the moles of oxygen needed to react completely with the glucose.
Conversion of the moles of required oxygen to grams, determining the mass of oxygen needed.
Use of mole ratios from the balanced equation to calculate the moles of carbon dioxide produced from glucose.
Calculation of the moles of water produced from the reaction, using the balanced chemical equation.
Conversion of moles of carbon dioxide to grams to find the mass of carbon dioxide produced.
Conversion of moles of water to grams to determine the mass of water produced in the reaction.
Conclusion summarizing the masses of oxygen required, carbon dioxide, and water produced from the reaction of 25 grams of glucose.
Transcripts
Browse More Related Video

Stoichiometry Basic Introduction, Mole to Mole, Grams to Grams, Mole Ratio Practice Problems

Step by Step Stoichiometry Practice Problems | How to Pass Chemistry
BTEC Applied Science: Unit 1 Chemistry Calculating Masses in Reactions

Classifying Types of Chemical Reactions Practice Problems
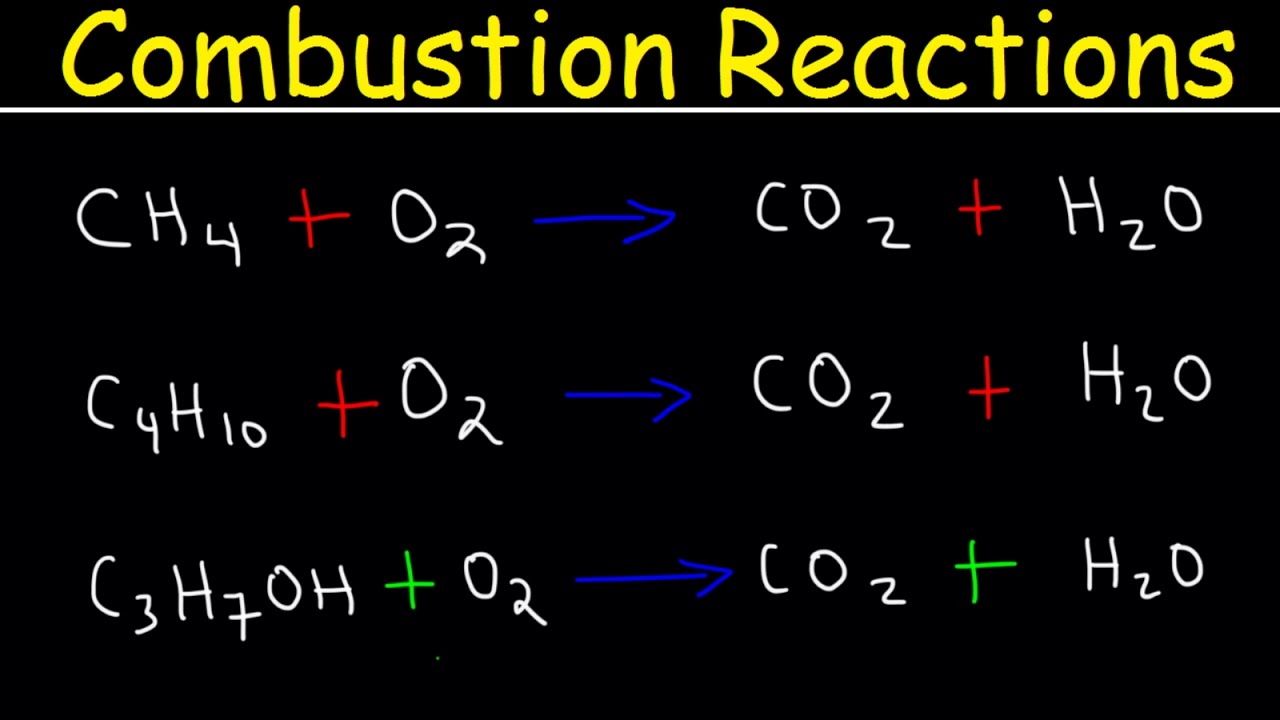
Balancing Combustion Reactions

Example of Finding Reactant Empirical Formula
5.0 / 5 (0 votes)
Thanks for rating: