How to find the number of Protons, Neutrons and Electrons? Chemistry
TLDRThis informative script teaches the fundamentals of identifying the number of protons, neutrons, and electrons in neutral atoms, ions, and isotopes. It explains the relationship between atomic and mass numbers, and how they determine the number of protons and neutrons. The script also covers how electrons are gained or lost to form ions, with examples of cations and anions, and concludes with a method for calculating the components of isotopes. The clear explanations and examples make it an engaging and educational resource.
Takeaways
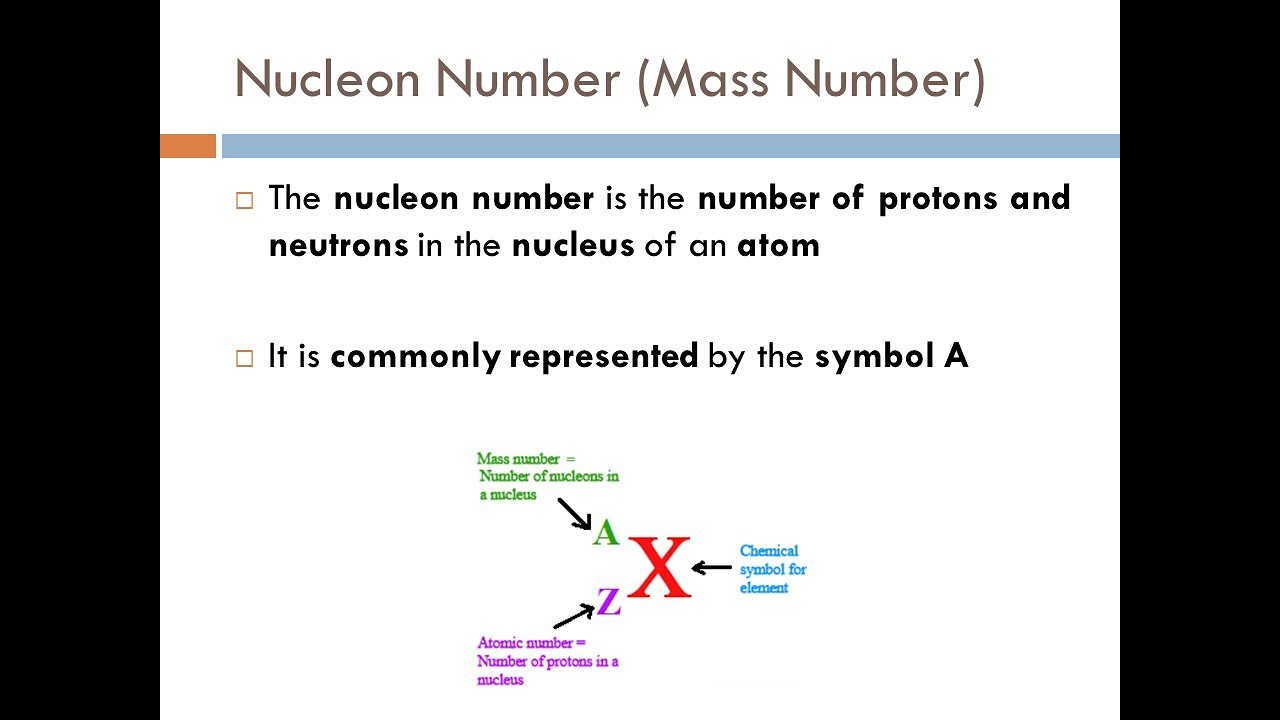
- 📚 The mass number of an atom is the sum of protons and neutrons, while the atomic number equals the number of protons.
- ⚛️ In a neutral atom, the number of protons equals the number of electrons.
- 👉 To find neutrons in a neutral atom, subtract the atomic number from the mass number.
- 🎈 For example, oxygen (O16) has 8 protons, 8 electrons, and (16-8)=8 neutrons.
- 💡 Sodium (Na) with a mass number of 23 and atomic number 11 has 11 protons, 11 electrons, and (23-11)=12 neutrons.
- 🌟 Aluminum (Al) with a mass number of 27 and atomic number 13 has 13 protons, 13 electrons, and (27-13)=14 neutrons.
- 🔋 Ions are charged atoms; cations (positive ions) lose electrons, and anions (negative ions) gain electrons.
- 💥 Aluminum ion (Al3+) has 13 protons, (27-13)=14 neutrons, and (13-3)=10 electrons.
- 🌱 Oxygen ion (O2-) has 8 protons, (16-8)=8 neutrons, and (8+2)=10 electrons.
- 🔍 In isotopes, the number of protons remains the same, but the number of neutrons varies, e.g., carbon isotopes have 6 protons and varying neutrons (carbon-12: 6 neutrons, carbon-13: 7 neutrons, carbon-14: 8 neutrons).
- 📈 The atomic number determines the element, while the mass number distinguishes isotopes of the same element.
Q & A
What is the mass number of a neutral atom of oxygen?
-The mass number of a neutral atom of oxygen is 16.
What is the atomic number of oxygen, and what does it represent?
-The atomic number of oxygen is 8, which represents the number of protons in the atom.
How many protons and electrons are in a neutral atom of oxygen?
-In a neutral atom of oxygen, there are 8 protons and 8 electrons.
How can you determine the number of neutrons in an atom?
-To determine the number of neutrons in an atom, subtract the atomic number from the mass number.
What is the mass number and atomic number of a sodium atom?
-The mass number of a sodium atom is 23, and the atomic number is 11.
How many protons, neutrons, and electrons are in an aluminum atom with a mass number of 27?
-In an aluminum atom with a mass number of 27, there are 13 protons, 14 neutrons, and 13 electrons.
What happens to the number of electrons in a cation compared to a neutral atom?
-In a cation, the number of electrons is less than in a neutral atom because positive ions lose electrons.
How does the number of electrons change in an anion compared to a neutral atom?
-In an anion, the number of electrons is more than in a neutral atom because negative ions gain electrons.
What is the relationship between the mass number and the number of neutrons in an atom?
-The number of neutrons in an atom is equal to the mass number minus the atomic number.
How many neutrons are in a chlorine atom with a mass number of 35?
-In a chlorine atom with a mass number of 35, there are 18 neutrons.
What are the differences in the number of neutrons among carbon-12, carbon-13, and carbon-14 isotopes?
-Carbon-12 has 6 neutrons, carbon-13 has 7 neutrons, and carbon-14 has 8 neutrons.
How can you identify the charge of an ion by looking at its symbol?
-A positive charge (cation) is indicated by a positive number, while a negative charge (anion) is indicated by a negative number next to the element symbol.
Outlines
🌟 Understanding Atomic Structure
This paragraph introduces the concepts of mass number and atomic number, using oxygen and sodium atoms as examples. It explains that the mass number is the sum of protons and neutrons, and the atomic number equals the number of protons. In a neutral atom, the number of protons equals the number of electrons. The paragraph also demonstrates how to calculate the number of neutrons by subtracting the atomic number from the mass number. It further illustrates these concepts with the examples of aluminum and chlorine atoms, emphasizing the relationship between the number of protons, electrons, and neutrons in neutral atoms.
🔋 Ions and Their Atomic Composition
This paragraph delves into the differences between cations and anions, explaining that cations are positive ions formed when an atom loses electrons, while anions are negative ions formed when an atom gains electrons. The paragraph uses the examples of aluminum and oxygen ions to illustrate how the number of electrons changes in ions compared to their neutral atoms, while the number of protons remains the same. It highlights the rule that positive charges in cations reduce the number of electrons and negative charges in anions increase the number of electrons. The paragraph concludes with a bonus tip on identifying the composition of isotopes, using carbon isotopes as examples.
Mindmap
Keywords
💡Protons
💡Neutrons
💡Electrons
💡Mass Number
💡Atomic Number
💡Ions
💡Isotopes
💡Neutral Atom
💡Cations
💡Anions
💡Periodic Table
Highlights
Understanding the basics of atomic structure is crucial for grasping chemical properties and reactions.
The mass number represents the sum of protons and neutrons in an atom.
The atomic number is equal to the number of protons, which also equals the number of electrons in a neutral atom.
In the case of oxygen (O16), there are 8 protons and 8 electrons, with the number of neutrons calculated as 16 - 8 = 8.
Sodium (Na) has a mass number of 23 and an atomic number of 11, indicating 11 protons and 12 neutrons.
Aluminum (Al) has a mass number of 27 and an atomic number of 13, with 13 protons and 14 neutrons.
Chlorine (Cl) with a mass number of 35 and an atomic number of 17 has 17 protons and 18 neutrons.
Ions are atoms or molecules that have gained or lost electrons, resulting in a net charge.
Cations are positively charged ions that have lost electrons, such as an aluminum ion (Al3+) which has 10 electrons.
Anions are negatively charged ions that have gained electrons, like the oxygen ion (O2-) with 10 electrons.
The charge on an ion indicates the number of electrons gained or lost, which is added to or subtracted from the neutral atom's electron count.
Isotopes are variants of an element with the same number of protons but different numbers of neutrons.
Carbon-12 (C-12) has 6 protons, 6 electrons, and 6 neutrons.
Carbon-13 (C-13) has 6 protons, 6 electrons, and 7 neutrons.
Carbon-14 (C-14) has 6 protons, 6 electrons, and 8 neutrons.
The method for calculating the number of protons, neutrons, and electrons in isotopes is similar to that for neutral atoms and ions.
This knowledge is essential for understanding radioactivity and the applications of isotopes in various fields.
The relationship between mass number, atomic number, and the number of protons, neutrons, and electrons is fundamental to chemistry and physics.
Transcripts
Browse More Related Video

Isotopes vs Ions | What is the Difference? |

Ions and Isotopes | Chemistry Animation

Atomic Number, Mass Number, and Net Electric Charge
Learning Outcomes (d) and (e) from Atomic Structure - JC H2 Chemistry
Protons, Neutrons and Electrons Explained - what's the difference?

GCSE Chemistry - Elements, Isotopes & Relative Atomic Mass #2
5.0 / 5 (0 votes)
Thanks for rating: