Colloids
TLDRColloids are intriguing mixtures that lie between homogeneous solutions and heterogeneous suspensions, characterized by their intermediate-sized particles. This video script delves into the properties of colloids, their interaction with light (Tyndall effect), and their classification into types such as aerosols, foams, gels, and emulsions. It highlights how colloids, unlike solutions, scatter light due to their larger particles, and unlike suspensions, do not settle over time or can be filtered.
Takeaways
- 🌟 Colloids are mixtures that exist between homogeneous solutions and heterogeneous suspensions, with particle sizes intermediate between the two.
- 📝 In a homogeneous solution, particles (atoms, ions, or small molecules) are very tiny and evenly distributed, giving a uniform appearance without settling over time.
- 🏖️ Heterogeneous mixtures, like sand in water, have larger particles that do not dissolve and can settle out over time, forming a suspension when stirred.
- 🌫️ Fog is an example of a colloid, consisting of tiny water droplets dispersed in air, which are large enough to scatter light but too small to settle out.
- 🔍 Colloids have two main parts: the dispersed substance (like water droplets in fog) and the dispersion medium (like air).
- 🌬️ Aerosols are a type of colloid where the dispersed substance is liquid or solid and the dispersion medium is a gas, such as fog (liquid in gas) or smoke (solid in gas).
- 💭 Foams are colloids with gas dispersed in a liquid medium, like whipped cream or shaving cream, and can also be formed by dispersing gas through a solid, as in marshmallows.
- 🍯 Gels are colloids where a solid is dispersed through a liquid, with examples including glue, paint, and gelatin, where the solid particles are suspended in the liquid medium.
- 🥚 Emulsions are colloids formed by two immiscible liquids (like oil and vinegar) that are held together by an emulsifying agent, such as egg yolk in mayonnaise.
- 💡 The Tyndall effect is a characteristic of colloids where they scatter light, making the light beam visible through the colloid, which is not seen in true solutions.
- 🚰 Filtration separates heterogeneous suspensions but not solutions or colloids, as the particles in colloids are too small to be caught by filters, similar to the particles in solutions.
Q & A
What is a colloid and how does it differ from a homogeneous and heterogeneous mixture?
-A colloid is a type of mixture that falls between a homogeneous mixture and a heterogeneous mixture. In a homogeneous mixture like a solution, the dissolved particles are very tiny and evenly distributed, giving a uniform appearance. In contrast, a heterogeneous mixture has distinct parts that don't dissolve and may form a suspension that settles over time. Colloids have intermediate-sized particles that are too small to settle but large enough to scatter light, making them visible.
What are the two main parts of a colloid?
-The two main parts of a colloid are the dispersed substance and the dispersion medium. The dispersed substance is the phase that is dispersed throughout the colloid, while the dispersion medium is the phase in which the dispersed substance is suspended.
Can you provide an example of a colloid and explain its properties?
-Fog is an example of a colloid. It consists of very tiny water droplets dispersed in air. These droplets are larger than molecules but too small to settle, allowing them to stay suspended. Fog exhibits the Tyndall effect, where it scatters light, making the light beam visible when shone through it.
What is an aerosol and what are some examples?
-An aerosol is a type of colloid where a liquid or solid is dispersed in a gas. Examples include fog (liquid droplets in air), smoke (solid particles in air), and aerosol sprays like disinfectant sprays (liquid in air).
What is the Tyndall effect and how does it relate to colloids?
-The Tyndall effect is the scattering of light by particles in a colloid. It is observed when a beam of light passes through a colloid and the path of the light becomes visible due to the scattering by the particles. This effect is a characteristic feature of colloids, distinguishing them from solutions where particles are too small to scatter light.
How does filtration work with colloids and solutions?
-Filtration separates mixtures based on the size of particles. In solutions, particles are too small to be retained by a filter and pass right through. Similarly, colloids have particles small enough to pass through a filter, preventing their separation by simple filtration. In contrast, heterogeneous suspensions with larger particles can be separated by filters as the particles stick to the filter paper.
What is a foam and how does it relate to colloids?
-Foam is a type of colloid where a gas is dispersed in a liquid medium. Examples include whipped cream, which has air bubbles dispersed in liquid cream, and shaving cream. Foams demonstrate the properties of colloids, with the dispersed gas bubbles being visible and contributing to the texture and stability of the mixture.
What is a gel and what are some common examples?
-A gel is a type of colloid where a solid is dispersed in a liquid. It forms a semi-solid substance with a jelly-like consistency. Common examples of gels include glue, paint, and gelatin. These substances have solid particles suspended in a liquid medium, giving them their characteristic properties.
What is an emulsion and how is it different from other colloids?
-An emulsion is a type of colloid where a liquid is dispersed in another liquid medium, typically two immiscible liquids that would not mix together, such as oil and water. Emulsions require an emulsifying agent to stabilize the mixture, like egg yolk in mayonnaise. Unlike other colloids, emulsions involve two liquids that would normally separate.
How do colloids demonstrate that nature isn't always easy to classify?
-Colloids show that nature isn't always easy to classify because they exhibit properties of both solutions and suspensions. They have intermediate-sized particles that are too small to settle like in a solution but large enough to scatter light like in a suspension. This dual nature makes colloids a complex and fascinating subject for scientific study.
What happens to heterogeneous suspensions over time?
-Heterogeneous suspensions, which consist of large particles that do not dissolve in the medium, will settle over time due to gravity. This separation occurs because the particles are large enough to overcome the randomizing effects of Brownian motion and eventually settle at the bottom of the container.
Outlines
🌫️ Introduction to Colloids and Mixtures
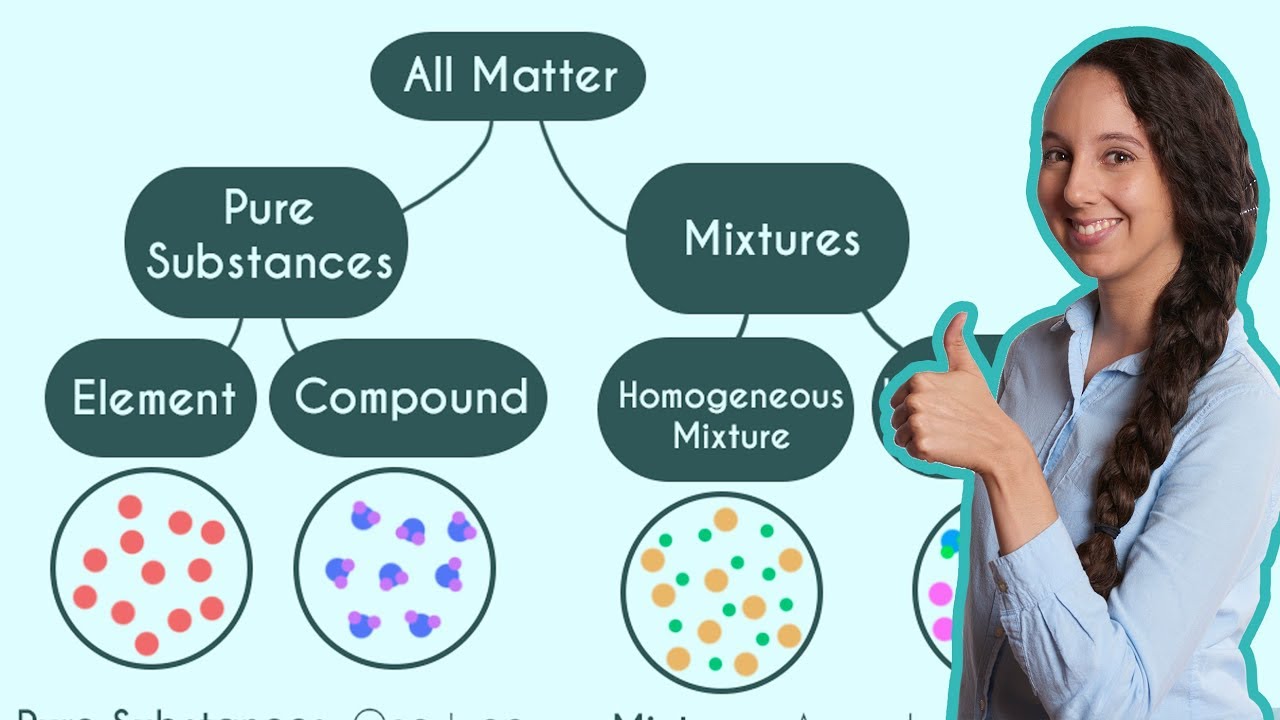
This paragraph introduces colloids as a type of mixture that lies between homogeneous and heterogeneous mixtures. It explains that colloids consist of particles that are larger than those in a solution but smaller than those in a suspension, preventing them from settling over time. The concept is illustrated using the example of water in the air, which forms a homogeneous mixture that can lead to the formation of raindrops or tiny droplets that create fog, a colloid. The paragraph also discusses the two main parts of a colloid: the dispersed substance and the dispersion medium, using fog as an example where water droplets are the dispersed substance and air is the dispersion medium.
🌿 Types of Colloids and Their Characteristics
This paragraph delves into the different types of colloids based on the dispersion medium and the dispersed substance. It explains aerosols, where liquid or solid is dispersed in a gas, using fog, mist, and smoke as examples. The paragraph then moves on to foams, which are colloids where gas is dispersed in a liquid, with whipped cream and shaving cream being common examples. Gels are discussed as a type of colloid where solid is dispersed in a liquid, with glue, paint, and gelatin mentioned. Emulsions, which are liquids dispersed in a liquid medium, are also covered, with mayonnaise and milk being typical examples. The paragraph concludes by highlighting the two phases present in all colloids and their general categories.
🔬 Interaction of Colloids with Light and Filtration
The final paragraph discusses the characteristics of colloids, focusing on their interaction with light, known as the Tyndall effect, where colloidal particles are large enough to scatter light, unlike the particles in a solution. This effect is used to differentiate colloids from solutions and suspensions. The paragraph also addresses the filtration of colloids, explaining that unlike suspensions, colloids cannot be separated by a filter paper because their particles are too small to be trapped. However, like solutions, the particles in colloids are small enough to pass through a filter, preventing the separation of the colloid into its components through filtration.
Mindmap
Keywords
💡Colloids
💡Homogeneous Mixture
💡Heterogeneous Mixture
💡Suspension
💡Aerosol
💡Foam
💡Gel
💡Emulsion
💡Dispersed Substance
💡Dispersion Medium
💡Tyndall Effect
💡Filtration
Highlights
Colloids are mixtures that fall between homogeneous and heterogeneous mixtures.
Homogeneous mixtures, like solutions, have very tiny particles that are evenly distributed.
Heterogeneous mixtures have distinct parts that do not dissolve and can settle over time.
Colloids are characterized by particles that are large enough to stay suspended but not large enough to settle.
Fog is an example of a colloid, consisting of tiny water droplets dispersed in air.
Colloids have two main parts called phases: the dispersed substance and the dispersion medium.
Aerosols are a type of colloid where liquid or solid is dispersed in a gas.
Foams are colloids with gas dispersed in a liquid medium, such as whipped cream or shaving cream.
Gels are colloids formed by dispersing solids in a liquid, like glue, paint, or gelatin.
Emulsions are colloids where a liquid substance is dispersed in a liquid medium, like oil and vinegar in salad dressing.
The Tyndall effect is the scattering of light by colloid particles, which is not seen in solutions.
Colloids, like solutions, have particles small enough to pass through filters, unlike heterogeneous suspensions.
The size of colloid particles is intermediate between those in solutions and heterogeneous suspensions.
Colloids demonstrate that nature is not always easily classified, offering a blend of solution and suspension properties.
The Tyndall effect can be observed in colloids due to the intermediate size of their particles.
Colloids show that mixtures can have characteristics of both solutions and suspensions, adding complexity to classification.
The study of colloids helps scientists understand the behavior of particles at intermediate sizes.
Colloids are interesting because they challenge the traditional classification of substances based on particle size and behavior.
Transcripts
Browse More Related Video

Solution, Suspension and Colloid | Is Matter around us pure? | Chemistry | Khan Academy

Mixtures - Class 9 Tutorial

Solution, Suspension and Colloid | #aumsum #kids #science #education #children

Solutions Overview and Types

Suspensions, colloids and solutions | Chemistry | Khan Academy
Pure Substances and Mixtures! (Classification of Matter)
5.0 / 5 (0 votes)
Thanks for rating: