Calculating Percent Composition and Empirical Formulas
TLDRIn this educational video, Mr. Causey from Atascocita, Texas, dives into the concepts of percent composition and empirical formulas used by chemists to determine the composition of unknown compounds. He explains the importance of the law of definite composition and multiple proportions, and guides viewers through calculating percentage composition, using molar masses to find empirical and molecular formulas. The video includes practical examples and encourages viewers to engage with the content by visiting Mr. Causey's website and YouTube channel for further learning.
Takeaways
- 🧪 Mr. Causey introduces a chemistry lesson on percent compositions and percentages from his virtual studio in Atascocita, Texas.
- 📚 The lesson covers important concepts for chemists, including percent by mass, empirical formulas, and molecular formulas.
- 🔢 Students are encouraged to use calculators and periodic tables to understand molar mass and chemical formula writing.
- 🔍 The law of definite composition and the law of multiple proportions are highlighted as foundational to understanding compound consistency.
- 🌟 The concept of percentage composition is introduced as the mass of an element in a compound expressed as a percentage of the total mass.
- ⚖️ Percent by mass is calculated by dividing the mass of an element by the total mass of the compound and multiplying by 100.
- 📉 To find the percentage of an element in a compound, multiply the total mass of the compound by the percentage of the element (converted to a decimal).
- 📚 Practice problems are provided, including calculating the percentage of hydrogen in HNO3 and determining how much iron can be obtained from rust (Fe2O3).
- 🔑 The process of finding an empirical formula involves assuming 100 grams of a compound, converting mass to moles, and using the smallest molar value to determine ratios.
- 🔍 To determine the molecular formula, calculate the mass of the empirical formula, divide the actual molar mass by the empirical formula mass, and adjust the empirical formula accordingly.
- 💻 Mr. Causey invites viewers to reach out with questions via email and to explore additional resources on his websites and YouTube channel.
Q & A
What is the main topic discussed in the video script?
-The main topic discussed in the video script is the concept of percent composition in chemistry, including percent by mass, empirical formulas, and molecular formulas.
What are the two laws mentioned in the script that help us understand the composition of compounds?
-The two laws mentioned are the law of definite composition and the law of multiple proportions, which were established in the eighteenth-century and help us understand that compounds always have the same ratio of elements.
What is the definition of percentage composition as discussed in the script?
-Percentage composition is the composition of a compound given in percentages, which can be used to determine the empirical and molecular formulas of different compounds.
How do you calculate the percent by mass of an element in a compound according to the script?
-To calculate the percent by mass, you take the mass of the element, divide it by the mass of the compound, and then multiply by 100.
What is the formula to find the mass of an element in a compound using percentage?
-The formula to find the mass of an element in a compound using percentage is the mass of the compound multiplied by the percent of the element (converted to a decimal).
What is the molar mass of HNO3 according to the script?
-The molar mass of HNO3, according to the script, is 63.02 grams.
How much hydrogen is in HNO3 in terms of percentage?
-The script calculates that there is approximately 1.61% hydrogen in HNO3 by mass.
What is the process to find the empirical formula of a compound with given mass percentages of elements?
-The process involves assuming 100 grams of the compound, converting the mass of each element to moles, and then writing a ratio using the smallest mole value as the denominator to determine the empirical formula.
How can you determine the molecular formula from the empirical formula and the molar mass of a compound?
-You determine the mass of the empirical formula, divide the actual molar mass by the empirical formula mass to find the ratio, and then multiply each element in the empirical formula by this ratio to get the molecular formula.
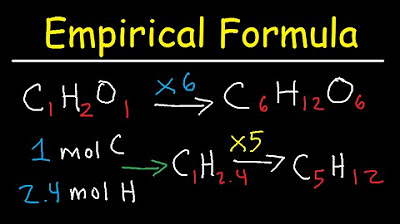
What is the empirical formula of a compound with 40.0% carbon, 6.70% hydrogen, and 53.3% oxygen?
-The empirical formula of the compound is CH2O, as determined by converting the mass percentages to moles and finding the simplest whole number ratio.
What is the molecular formula of a compound if the empirical formula is CH2O and the molar mass is 60.06 grams?
-The molecular formula is C2H4O2, obtained by multiplying the empirical formula by 2, which is the ratio of the actual molar mass to the empirical formula mass.
Outlines
🧪 Chemistry of Percent Composition
In this video script, Mr. Causey introduces the concept of percent composition, a fundamental tool in chemistry for determining the composition of unknown compounds. He emphasizes the importance of understanding the law of definite composition and the law of multiple proportions, which establish that compounds have a consistent ratio of elements. The script explains how to calculate the percentage composition of a compound by mass, using the mass of an element in the compound and the total mass of the compound. An example is provided to illustrate the calculation of the percentage of hydrogen in HNO3. The lesson also covers the process of determining empirical and molecular formulas using percentage composition, with a step-by-step example included.
📚 Calculating Empirical and Molecular Formulas
This section of the script delves into the practical application of percent composition to determine empirical and molecular formulas of compounds. Mr. Causey demonstrates how to find the molar mass of compounds and convert mass percentages to moles using the periodic table. He guides viewers through the process of calculating the percentage of iron in Fe2O3 (rust) and uses this information to determine the empirical formula of a compound with given mass percentages of carbon, hydrogen, and oxygen. The script also explains how to find the molecular formula when the molar mass of the compound is known, by comparing it to the empirical formula's mass and adjusting accordingly. The example concludes with the calculation of the molecular formula for a compound with a molar mass of 60.06 grams.
📧 Recap and Additional Resources
In the final paragraph, Mr. Causey recaps the key points covered in the lesson, including the calculation of percentage composition, the determination of empirical and molecular formulas, and the importance of using the periodic table and calculator for these processes. He invites viewers to reach out with questions via email and encourages them to visit his websites for additional resources such as PowerPoint videos. Mr. Causey also promotes his YouTube channel, humorously suggesting that subscribing could increase one's IQ.
Mindmap
Keywords
💡Percent Composition
💡Molar Mass
💡Law of Definite Composition
💡Law of Multiple Proportions
💡Empirical Formula
💡Molecular Formula
💡Percentage by Mass
💡Periodic Table
💡Calculator
💡Chemical Formulas
💡Significant Figures
Highlights
Introduction to the topic of percent compositions and percentages in chemistry.
Importance of understanding the composition of unknown compounds for empirical lessons in chemistry.
Necessity of having a calculator and periodic table for the lesson.
Explanation of concepts like percent by mass, empirical formulas, and molecular formulas.
The law of definite composition and the law of multiple proportions as foundational to understanding compounds.
How to calculate percentage composition using mass of an element and mass of the compound.
Practice problem involving calculating the percentage of hydrogen in HNO3.
Method to determine molar masses and use them to find percentages in compounds.
Example of calculating how much iron can be obtained from rust (Fe2O3).
Explanation of the process to find the empirical formula of a compound given its composition.
Conversion of mass to moles using the periodic table for empirical formula calculation.
How to determine the molecular formula from the empirical formula and a given molar mass.
Step-by-step calculation for empirical and molecular formulas using a specific compound as an example.
Recap of the lesson's main points including percentage composition and formula calculations.
Invitation to contact the instructor for questions and additional resources.
Promotion of additional educational resources and the instructor's YouTube channel.
Transcripts
Browse More Related Video
Writing Empirical Formulas From Percent Composition - Combustion Analysis Practice Problems
Empirical Formula and Molecular Formula | Basic Concept | Numerical Problems

Find the Empirical Formula Given Percents

Molecular and Empirical Forumlas from Percent Composition

Empirical Formula & Molecular Formula Determination From Percent Composition

Determining the Empirical Formula from a Percent
5.0 / 5 (0 votes)
Thanks for rating: