Writing Empirical Formulas From Percent Composition - Combustion Analysis Practice Problems
TLDRThis educational video script offers a comprehensive guide on determining empirical and molecular formulas from mass percent composition and combustion analysis. It explains the process of simplifying molecular formulas to empirical ones, converting grams of elements to moles, and using ratios to find whole number subscripts. The script also demonstrates how to calculate molar masses and scale empirical formulas to molecular formulas, providing step-by-step solutions to several chemistry problems involving carbon, hydrogen, and oxygen.
Takeaways
- 🔍 The empirical formula represents the simplest whole number ratio of elements in a compound.
- 🧪 To find the empirical formula from mass percent composition, convert percentages to grams and then to moles, followed by dividing by the smallest number of moles to get whole number ratios.
- ⚖️ The molar mass of an element is crucial for converting grams to moles, using the atomic mass from the periodic table.
- 🔢 If the moles of elements do not result in whole numbers after division, multiply by a factor that will convert the decimals into whole numbers, often related to fractions.
- 🔬 In combustion analysis, the mass of CO2 and H2O produced can be used to determine the moles of carbon and hydrogen in the original compound.
- 🔄 The moles of carbon in CO2 and the moles of hydrogen in H2O are equal to the moles of these elements in the compound, but oxygen must be calculated differently due to its presence in the air.
- 📉 To find the molecular formula from the empirical formula, calculate the molar mass of the empirical formula and compare it to the molar mass of the molecular formula, then adjust the subscripts accordingly.
- 🌐 The empirical formula can be used to find the molecular formula if additional information, such as the molar mass of the molecular formula, is provided.
- 🌡️ For compounds containing carbon, hydrogen, and oxygen, the mass of oxygen in the compound must be determined by subtracting the masses of carbon and hydrogen from the total mass.
- 📝 When dealing with combustion analysis involving three elements, convert the moles of carbon and hydrogen to grams, then find the mass of oxygen and convert it to moles to determine the empirical formula.
- 📚 The script provides a systematic approach to finding empirical and molecular formulas using mass percent, molar mass, and combustion analysis.
Q & A
What is an empirical formula in chemistry?
-An empirical formula is the simplest whole-number ratio of atoms or ions in a compound. It represents the lowest ratio of subscripts in a chemical formula.
How can you find the empirical formula of a compound given its molecular formula?
-You can find the empirical formula by simplifying the molecular formula's subscripts to the lowest whole number ratios. For example, if the molecular formula is C3H9O6, dividing each subscript by 3 gives the empirical formula CH3O2.
What is the first step in determining the empirical formula from the mass of elements in a compound?
-The first step is to convert the mass of each element in grams into moles using their respective atomic masses (molar masses).
How do you convert grams of an element into moles?
-You convert grams into moles by dividing the mass of the element in grams by its atomic mass (molar mass). For example, to convert grams of carbon into moles, divide the grams by 12, since the molar mass of carbon is 12 grams per mole.
What is the next step after converting the mass of elements into moles?
-After converting to moles, you divide each number by the smallest mole value to find the whole number ratio of atoms, which gives you the empirical formula.
How can you find the molecular formula from the empirical formula if you know the molar mass of the molecular formula?
-You calculate the molar mass of the empirical formula and then divide the molar mass of the molecular formula by the molar mass of the empirical formula. The result is the factor by which you multiply the subscripts of the empirical formula to get the molecular formula.
What is the process of finding the empirical formula from percent composition?
-You convert the percent composition of each element to grams based on a 100-gram sample of the compound. Then, convert these grams into moles and follow the process of finding the empirical formula as with the mass method.
How do you handle non-whole number ratios when determining the empirical formula from moles?
-You find a whole number that, when multiplied by the non-whole number ratio, results in a whole number. This is done by recognizing the fraction's simplest form or systematically multiplying by integers until a whole number is achieved.
What is a combustion analysis problem in the context of finding an empirical formula?
-A combustion analysis problem involves determining the empirical formula of a compound by measuring the mass of CO2 and H2O produced during combustion, which indicates the amount of carbon and hydrogen in the original compound.
How do you find the empirical formula of a compound containing carbon, hydrogen, and oxygen from combustion analysis?
-You determine the moles of carbon and hydrogen from the mass of CO2 and H2O produced. Then, calculate the mass and moles of oxygen in the original compound by subtracting the mass of carbon and hydrogen from the total mass and converting it to moles. Finally, divide by the smallest mole value to find the empirical formula.
Outlines
🔍 Introduction to Empirical Formulas
This paragraph introduces the concept of empirical formulas in chemistry. It explains that the empirical formula represents the simplest whole number ratio of elements in a compound. The script provides examples of simplifying complex molecular formulas to their empirical forms, such as reducing C3H9O6 to CH3O2 by dividing by three. It also sets up the process for finding the empirical formula from the mass of elements given in a compound, converting mass to moles, and then simplifying the mole ratio to the lowest whole numbers.
🧪 Converting Mass Percent to Empirical Formula
The second paragraph delves into using mass percent composition to determine an empirical formula. It illustrates the process of converting mass percentages of elements to grams, then to moles, and finally to the empirical formula by dividing by the smallest number of moles to get whole number ratios. The script also addresses the challenge of dealing with non-whole number ratios by adjusting the subscripts to achieve whole numbers, using the example of a compound with 81.8% carbon and 18.2% hydrogen.
🔬 Combustion Analysis to Determine Empirical Formulas
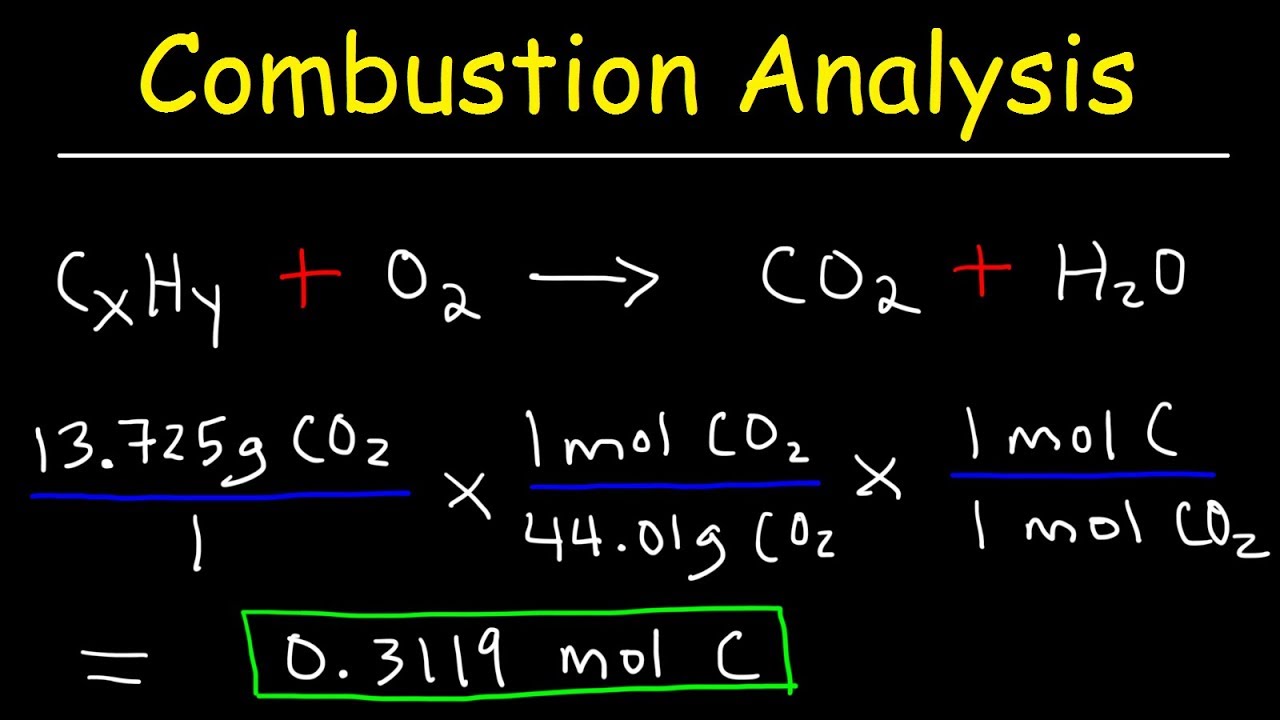
This paragraph focuses on combustion analysis, explaining how to find the empirical formula of a compound containing only carbon and hydrogen based on the mass of CO2 and H2O produced. The script details the steps of converting the mass of CO2 and H2O to moles of carbon and hydrogen, respectively, and then determining the empirical formula by dividing these moles by the smallest number to achieve whole numbers. It also introduces a method for finding the molecular formula from the empirical formula using molar mass.
📚 Advanced Combustion Analysis with Three Elements
The fourth paragraph extends the combustion analysis to include a compound with carbon, hydrogen, and oxygen. It explains the importance of distinguishing between the oxygen from the compound and the oxygen from the air that is incorporated into CO2 and H2O during combustion. The script outlines the process of finding the moles of carbon and hydrogen from CO2 and H2O, converting these moles to grams, and then determining the mass of oxygen in the original compound. It concludes with the method for finding the empirical formula by dividing the moles of each element by the smallest number of moles.
🔄 Determining Empirical Formulas from Combustion Data
This paragraph continues with the theme of combustion analysis but adds complexity by including a compound with three elements. It emphasizes the need to find the moles of carbon and hydrogen from the combustion products and then to calculate the moles of oxygen differently, as oxygen from the air contributes to the combustion products. The script provides a step-by-step method for converting moles of elements to grams, finding the mass of oxygen in the compound, and then determining the empirical formula by normalizing the moles of each element.
🧐 Final Thoughts on Empirical Formulas
The final paragraph wraps up the discussion on empirical formulas, summarizing the process and emphasizing the importance of simplifying ratios to whole numbers. It provides a systematic approach to finding the correct multiplier for non-whole number ratios and highlights the pattern recognition involved in converting decimals to whole numbers by multiplying by appropriate integers. The script concludes with a reminder of the method for finding the empirical formula from combustion data and thanks the viewer for watching.
Mindmap
Keywords
💡Empirical Formula
💡Molar Mass
💡Percent Composition
💡Combustion Analysis
💡Moles
💡Molecular Formula
💡Divisibility
💡Ratio
💡Subscripts
💡Systematic Approach
Highlights
Introduction to finding empirical formulas from mass percent composition and combustion analysis.
Explanation of empirical formula as the simplest whole number ratio of elements in a compound.
Demonstration of simplifying a molecular formula to its empirical formula by dividing by the greatest common divisor.
Method to convert grams of elements into moles using atomic masses for empirical formula determination.
Process of determining empirical formula from given masses of elements in a compound.
Conversion of moles of elements to whole number ratios to find the empirical formula.
Technique to find the molecular formula from the empirical formula using molar mass.
Approach to determine empirical formula from percent composition of elements.
Conversion of mass percent to grams for calculation in empirical formula determination.
Dealing with non-whole number ratios by multiplying by a whole number to achieve whole number subscripts.
Pattern recognition for converting decimals to whole numbers in empirical formula calculations.
Combustion analysis problem solving to find the empirical formula of a compound containing carbon and hydrogen.
Use of molar mass of CO2 and H2O to determine moles of carbon and hydrogen in a compound.
Systematic method to find the appropriate multiplier for subscripts to achieve whole numbers.
Combustion analysis involving compounds with three elements (C, H, O) and how to handle oxygen separately.
Strategy for finding the mass of oxygen in a compound by subtracting the masses of carbon and hydrogen from the total mass.
Final empirical formula determination for a compound with carbon, hydrogen, and oxygen using moles and mass conversion.
Conclusion summarizing the process and methods for finding empirical and molecular formulas from various types of analysis.
Transcripts
Browse More Related Video
Introduction to Combustion Analysis, Empirical Formula & Molecular Formula Problems

Empirical Formula & Molecular Formula Determination From Percent Composition

Molecular and Empirical Forumlas from Percent Composition

Molecular and Empirical Formulas

Empirical and Molecular Formulas from Stoichiometry

Calculating Empirical Formulas with Percent Composition
5.0 / 5 (0 votes)
Thanks for rating: