How To Write Chemical Equations From Word Descriptions
TLDRThis video tutorial explains how to write and balance chemical equations from word descriptions. It covers several examples, including the reactions of phosphorus with fluorine, calcium with nitrogen, sulfur with oxygen, and others. The tutorial emphasizes the importance of understanding diatomic molecules, ionic compounds, the solubility rules, and different types of chemical reactions. It also demonstrates methods like the crisscross technique for writing formulas and offers tips for balancing equations. The video aims to help viewers grasp these fundamental concepts in chemistry, providing links to additional resources for further learning.
Takeaways
- 📝 The video instructs how to convert word descriptions into balanced chemical equations.
- 🔬 It emphasizes the importance of recognizing the states of elements like phosphorus, fluorine, nitrogen, and others in reactions.
- 🔄 The process of balancing chemical equations involves ensuring equal numbers of atoms for each element on both sides of the equation.
- ⚛️ The video explains the method to balance equations by adjusting coefficients for elements like phosphorus in the reaction with fluorine to form PF5.
- 🧪 It details the steps to write chemical formulas for compounds, especially ionic compounds, using the crisscross method for charge balance.
- 🌐 The video mentions the need for knowledge of solubility rules to determine the state of products in reactions, such as whether barium chloride is soluble in water.
- ⚔️ It discusses the prediction of products in reactions, especially for double replacement reactions, and the importance of understanding different types of chemical reactions.
- 🔢 The script provides examples of balancing equations for various reactions, including single replacement and combustion reactions.
- 🔥 In the context of combustion reactions, the script advises to balance carbon atoms first, followed by hydrogen, and then oxygen last.
- 🛠️ The video script also touches on the activity series of metals, which is crucial for predicting outcomes in single replacement reactions.
- 📚 Additional resources such as links to other videos on solubility rules, predicting reaction products, and types of chemical reactions are provided for further learning.
Q & A
What is the main topic of the video?
-The main topic of the video is teaching how to write and balance chemical equations from word descriptions.
What are the seven diatomic elements mentioned in the video?
-The seven diatomic elements mentioned are nitrogen (N2), oxygen (O2), fluorine (F2), chlorine (Cl2), bromine (Br2), iodine (I2), and hydrogen (H2).
What is the product of the reaction between solid elemental phosphorus and fluorine gas?
-The product of the reaction between solid elemental phosphorus (P4) and fluorine gas (F2) is phosphorous pentafluoride (PF5), which is a gas.
How do you balance the chemical equation for the reaction between phosphorus and fluorine?
-To balance the equation, first multiply PF5 by 4 to have 4 phosphorus atoms on the right side, then multiply F2 by 10 to have 20 fluorine atoms on the left side, ensuring the balance of both elements.
What is the formula for calcium nitride and why is it written that way?
-The formula for calcium nitride is Ca3N2. It is written that way because calcium has a +2 charge and nitride has a -3 charge. Using the crisscross method, the charges become the subscripts, resulting in the formula Ca3N2 for charge balance.
What is the difference between ionic and covalent compounds in terms of formula writing?
-Ionic compounds are formed between a metal and a nonmetal and are written using the crisscross method to balance charges, while covalent compounds are formed between nonmetals and are written by simply combining the elements with appropriate subscripts if necessary.
What is the general phase of most ionic compounds?
-Most ionic compounds are generally in the solid phase at room temperature.
How do you balance the chemical equation for the reaction between sulfur and oxygen to form sulfur trioxide?
-Start by balancing the sulfur atoms by putting an 8 in front of SO3. Then balance the oxygen atoms by putting a 12 in front of O2, ensuring there are 24 oxygen atoms on both sides of the equation.
What is the chemical formula for hydrochloric acid and why is it soluble in water?
-The chemical formula for hydrochloric acid is HCl. It is soluble in water because most acids are soluble, especially when they are part of the aqueous phase.
What is the process for balancing a chemical equation in a double replacement reaction?
-In a double replacement reaction, you balance the equation by first balancing the cations and then the anions, ensuring that the total positive and negative charges are equal on both sides of the equation.
What is the expected product of a combustion reaction and how should you balance it?
-The expected products of a combustion reaction are always carbon dioxide (CO2) and water (H2O). To balance it, start by balancing the carbon atoms, then the hydrogen atoms, and finally the oxygen atoms last.
What is the activity series and why is it important in predicting the products of a single replacement reaction?
-The activity series is a list of metals arranged in order of decreasing reactivity. It is important in predicting the products of a single replacement reaction because a more reactive metal can displace a less reactive metal from its compound.
Outlines
📚 Introduction to Writing and Balancing Chemical Equations
This paragraph introduces the process of converting word descriptions into balanced chemical equations. It begins with an example of elemental phosphorus (P4) reacting with fluorine gas (F2) to form phosphorus pentafluoride (PF5). The importance of recognizing diatomic elements and the states of matter is emphasized. The paragraph demonstrates the step-by-step method of balancing the equation by ensuring equal numbers of phosphorus and fluorine atoms on both sides of the equation.
🔍 Writing Formulas for Ionic Compounds and Balancing Equations
The second paragraph delves into the specifics of writing chemical formulas for ionic compounds, using calcium metal (Ca) reacting with nitrogen gas (N2) to form calcium nitride as an example. It explains the concept of valence electrons and charges, and introduces the crisscross method for determining the subscripts in the formula. The paragraph also highlights the need to balance the charges in ionic compounds and demonstrates how to balance the chemical equation by adjusting the coefficients.
🌐 Understanding Ionic Compounds and Predicting States of Matter
This paragraph discusses the characteristics of ionic compounds, which are typically formed between metals and nonmetals, and their usual state as solids. It provides guidance on how to determine the physical state of a compound when it's not explicitly given, suggesting that most ionic compounds are solids. The paragraph also includes the process of balancing a chemical equation for a reaction involving sulfur and oxygen, emphasizing the distinction between ionic and covalent compounds.
🧪 Solubility Rules and Predicting Products of Chemical Reactions
The fourth paragraph focuses on solubility rules, particularly for ionic compounds, and the importance of knowing the solubility of different compounds in water. It uses the reaction between hydrochloric acid and barium hydroxide to illustrate the process of writing and balancing chemical equations, including the use of parentheses for polyatomic ions. The paragraph also touches on the need to predict products in double replacement reactions and provides resources for learning about different types of chemical reactions.
🔄 Balancing Single Replacement Reactions and Activity Series
This paragraph explores single replacement reactions, using the reaction between zinc metal and hydrobromic acid as an example. It explains the process of balancing such reactions by focusing on the conservation of atoms, starting with the metal and then the nonmetal, and finally adjusting for the hydrogen atoms. The paragraph also introduces the concept of the activity series, which is crucial for predicting the feasibility of reactions involving metals.
🔥 Balancing Combustion Reactions and Understanding Solubility
The final paragraph discusses combustion reactions, specifically the burning of pentane in air to produce water and carbon dioxide. It outlines the strategy for balancing these reactions, which typically involves balancing carbon atoms first, then hydrogen, and finally oxygen. The paragraph also emphasizes the importance of understanding solubility to correctly write the phase of products in chemical equations, and it invites viewers to explore additional resources for further learning.
Mindmap
Keywords
💡Chemical Equation
💡Diatomic Molecule
💡Balancing Equations
💡Ionic Compound
💡Solubility Rules
💡Polyatomic Ion
💡Crisscross Method
💡Activity Series
💡Combustion Reaction
💡Double Replacement Reaction
Highlights
Introduction to writing chemical equations from word descriptions and balancing them.
Explanation of how to handle elemental phosphorus (P4) and fluorine gas (F2) in chemical equations.
Identification of diatomic elements like nitrogen, oxygen, fluorine, chlorine, bromine, and iodine.
Conversion of word description into a chemical equation for phosphorous pentafluoride (PF5).
Balancing the chemical equation by focusing on phosphorus and fluorine atoms.
Writing chemical formulas for ionic compounds, demonstrated with calcium nitride.
Use of the crisscross method to determine the formula of calcium nitride (Ca3N2).
Understanding the solubility of ionic compounds and predicting their state (solid, liquid, gas).
Balancing the chemical equation for the reaction between calcium metal and nitrogen gas.
Writing and balancing a chemical equation for sulfur and oxygen gas to form sulfur trioxide.
Differentiating between covalent and ionic compounds and their respective formulas.
Balancing the chemical equation for the reaction between hydrochloric acid and barium hydroxide.
Importance of knowing solubility rules for predicting the state of products in chemical reactions.
Balancing a single replacement reaction between zinc metal and hydrobromic acid.
Understanding the activity series and its role in predicting the products of single replacement reactions.
Balancing a combustion reaction for pentane, emphasizing the order of balancing elements.
Conclusion summarizing the process of writing and balancing chemical equations from word descriptions.
Transcripts
Browse More Related Video

Combination Reactions

HOW TO FIGURE OUT THE STATE OF AN ELEMENT OR COMPOUND | EASY
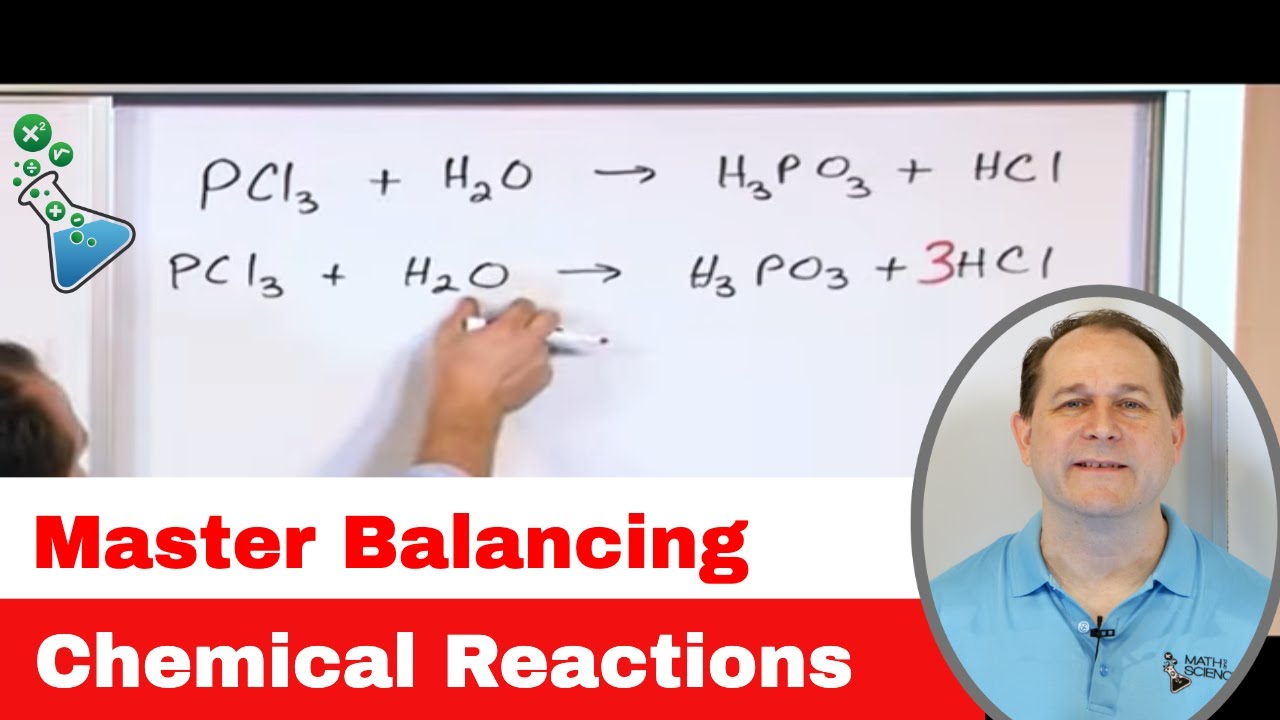
Mastering the Art of Balancing Chemical Reactions in Chemistry

BTEC Applied Science: Unit 1 Chemistry Elements

Writing Chemical Formulas For Covalent Molecular Compounds

Chemical Reactions - Combination, Decomposition, Combustion, Single & Double Displacement Chemistry
5.0 / 5 (0 votes)
Thanks for rating: