Ep9 Cationic and Anionic Polymerization - UC San Diego - NANO 134 Darren Lipomi
TLDRIn this chemistry lecture, the instructor delves into the complexities of ionic polymerization, a key method of chain growth polymerization. They explain cationic and anionic polymerization, highlighting the differences in charge and stability. The lecture covers the formation of polyolefins and ring-opened polymers, emphasizing the living nature of anionic polymerization and its sensitivity to water. The role of electron-rich and electron-poor groups in stabilizing charges and the impact of solvent dielectric constants on reaction rates are also discussed, providing a comprehensive overview of polymerization processes.
Takeaways
- 📘 The course is transitioning from organic chemistry to thermodynamics, focusing on the physical chemical principles of polymers.
- 🔬 Ionic polymerization is introduced as a key method of chain growth polymerization, producing products similar to those from radical polymerization but mediated by electron pairs.
- ⚛️ Cationic polymerization involves a positive charge on the active center of the polymer chain, while anionic polymerization involves a negative charge.
- 🔄 Anionic polymerization is described as 'living' because it lacks the possibility of termination by combination or disproportionation, unlike radical polymerization.
- 🌐 The stability of carbocations in cationic polymerization is influenced by the ability of surrounding atoms to donate electron density, favoring secondary and tertiary over primary carbons.
- 💧 Anionic polymerization is highly sensitive to water, which can terminate the reaction, necessitating careful handling and anhydrous conditions in the lab.
- 🔑 The role of the counter ion in ionic polymerization is crucial; larger ions diffuse the negative charge and are less tightly bound, increasing the propagation rate.
- 🌡️ The dielectric constant of the solvent plays a significant role in the reaction rate of cationic polymerization, with higher constants leading to faster reactions by reducing electrostatic interactions.
- 🔍 The script discusses the importance of electron-rich (donating) and electron-poor (withdrawing) substituents in stabilizing charges during polymerization.
- 🔗 The kinetic chain length in anionic polymerization is equivalent to the number average degree of polymerization due to the absence of termination by combination.
- 📚 Additional topics such as ring-opening polymerization and the use of alkyl lithium reagents in anionic polymerization are covered, highlighting the ability to create complex polymeric structures.
Q & A
What is the main focus of the lecture for the day and Wednesday?
-The main focus of the lecture is on ionic polymerization, which is a key method of chain growth polymerization, and it will be the last topic related to chemical structures before moving on to thermodynamics.
What are the two types of ionic polymerization discussed in the script?
-The two types of ionic polymerization discussed are cationic polymerization, where there is a positive charge on the active center, and anionic polymerization, where there is a negative charge attacking the next monomer.
Why are anionic polymerizations considered 'living'?
-Anionic polymerizations are considered 'living' because they can continue to add monomers indefinitely without termination by combination or disproportionation, unlike radical polymerization.
What is the role of the counter ion in ionic polymerization?
-The counter ion, or the nonreactive conjugate base, is important because larger ions can diffuse the negative charge around their large bulk, allowing them to be less tightly bound to the growing chain end, which increases the propagation rate constants.
How does the dielectric constant of a solvent affect the rate of cationic polymerization?
-A high dielectric constant solvent can screen charges more effectively, allowing the counter ion and the growing chain end to be farther apart, which in turn increases the reaction rate by allowing more monomer molecules to add to the growing chain.
What is the significance of the kinetic chain length in ionic polymerization?
-The kinetic chain length is significant because it represents the number of monomers added per unit time over the number of chains formed per unit time, and it is equal to the number average degree of polymerization in ionic polymerization.
What is the difference between anionic and cationic polymerization in terms of control over the polymerization process?
-Anionic polymerization is more controlled because it can be terminated easily by the presence of water or water vapor, allowing for precise control over the reaction. Cationic polymerization, on the other hand, can terminate by combination or disproportionation, which can lead to less controlled polymerization.
Why is the presence of water critical in anionic polymerization?
-The presence of water is critical in anionic polymerization because even trace amounts of water can terminate the reaction by neutralizing the negative charge at the growing polymer chain end.
What are some examples of monomers that can undergo ring-opening polymerization?
-Examples of monomers that can undergo ring-opening polymerization include ethylene oxide, ethylene amine, and tetrahydrofuran.
How does the polarity of the solvent influence the rate of anionic polymerization?
-The polarity of the solvent increases the rate of anionic polymerization because it allows the counter ion to dissociate farther from the growing chain end, enabling more monomers to add to the chain.
What is the role of electron-donating or electron-withdrawing groups in the polymerization process?
-In cationic polymerization, electron-donating groups help stabilize the positive charge on the growing chain end. In anionic polymerization, electron-withdrawing groups make the carbon atom more receptive to attack by negatively charged species, facilitating the propagation of the polymer chain.
Outlines
📚 Introduction to Week 4 of Nanoscience Chemistry 134
The instructor begins by welcoming students to the fourth week of the Nanoscience Chemistry 134 course, expressing hope that they are still engaged after the exam. The focus for the week is on chemical structures, with an emphasis on ionic polymerization, a key method of chain growth polymerization. The lecture will cover the differences between cationic and anionic polymerization, which are mediated by pairs of electrons instead of single electrons like in radical polymerization. The instructor promises a transition from organic chemistry to thermodynamics, discussing how the theory of two solvents translates to polymers and their molecular structure, ultimately affecting their thermo mechanical behavior.
🔬 Understanding Ionic Polymerization and Living Polymers
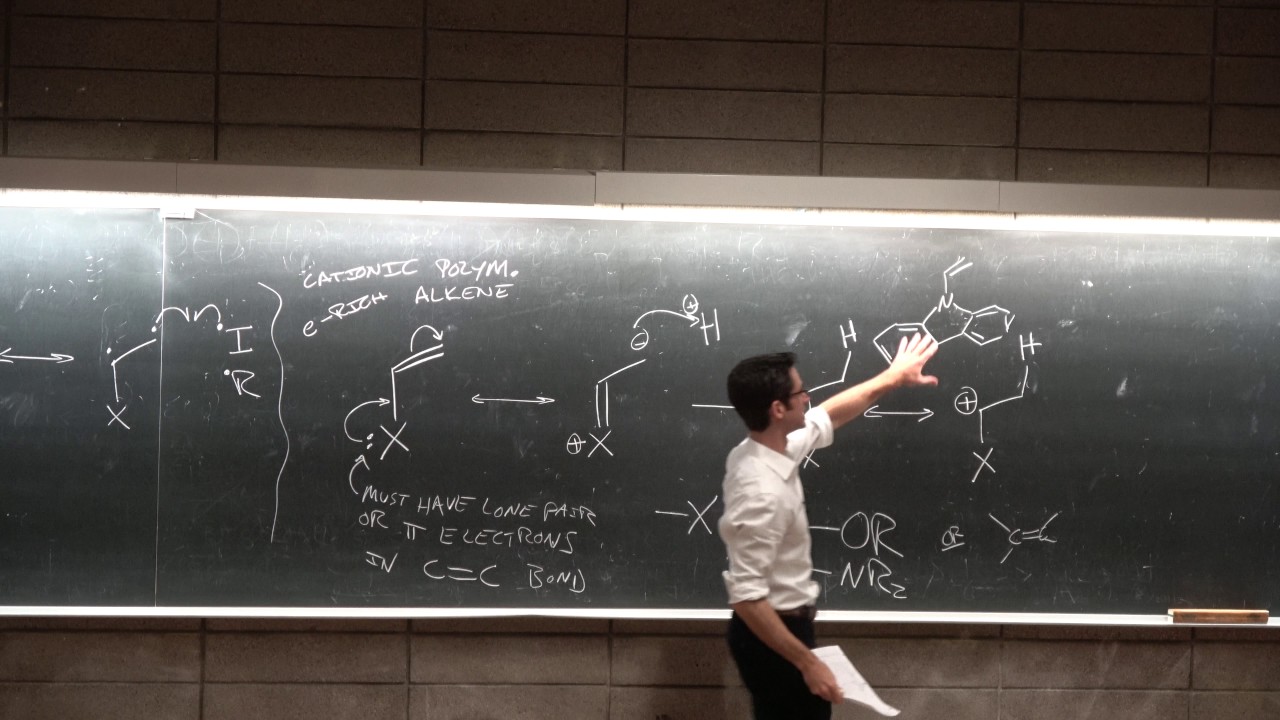
The summary delves into the specifics of ionic polymerization, contrasting it with radical polymerization. The instructor explains that anionic polymerization is considered 'living' because it lacks termination steps like combination or disproportionation, which are common in radical polymerization. The concept of formal charge is reintroduced, and the stability of carbocations is discussed, with secondary and tertiary cations being more stable than primary ones. The role of the conjugate base in cationic polymerization is highlighted, emphasizing the need for a non-reactive, delocalized charge. The summary also touches on the importance of the solvent and the dielectric constant in influencing the propagation rate of the polymerization reaction.
🌐 The Role of Dielectric Constant in Polymerization
This paragraph explores the impact of the solvent's dielectric constant on the polymerization process. A high dielectric constant allows for better charge screening, reducing the electrostatic attraction between the growing polymer chain and the counter ion. This results in a faster reaction rate as monomers can more easily add to the chain. The instructor provides a detailed example of how varying the dielectric constant by adjusting the solvent composition significantly increases the reaction rate constant. The summary also explains the kinetic chain length and its relation to the degree of polymerization in the context of ionic polymerization.
🔄 Ring-Opening Polymerization and Its Mechanism
The instructor introduces ring-opening polymerization (ROP), a process that allows for the formation of polymers with heteroatoms along the chain. The mechanism involves the use of an initiator, which can be a proton or the growing chain end, and the attack of the oxygen's lone pair on this positive charge. The summary explains how the positive charge becomes delocalized, leading to the opening of the ring and the formation of polyethylene oxide. The paragraph also mentions the potential for resonance structures and the importance of understanding the reactivity of the carbon atom in the polymerization process.
🚫 Sensitivity of Anionic Polymerization to Water
Anionic polymerization is highlighted as a highly useful method for creating a variety of polymeric structures, including block copolymers. However, it is extremely sensitive to water, which can terminate the reaction. The process typically begins with an alkyl lithium reagent, such as butyl lithium, which is noted for its potential dangers in the lab. The instructor emphasizes the need for a dry environment and the use of less reactive lithium reagents for safety. The summary also discusses the propagation step in anionic polymerization and the importance of quenching the reaction with water to terminate the chains.
🔄 Termination of Cationic and Anionic Polymerization
The instructor contrasts the termination steps of cationic and anionic polymerization. Cationic polymerization can terminate through unimolecular or bimolecular pathways, involving the loss of a hydrogen atom or the transfer of a proton to another monomer. In contrast, anionic polymerization is described as 'living' and can only be terminated by the addition of water. The summary explains that anionic polymerization maintains reactive chain ends, allowing for the potential creation of block copolymers and other complex structures once more monomers are added.
🌡 The Influence of Solvent Polarity on Anionic Polymerization
The role of solvent polarity in anionic polymerization is emphasized, with the rate of polymerization increasing with the polarity of the solvent. This is attributed to the ability of polar solvents to allow the counter ion to dissociate farther from the growing chain end, facilitating the addition of more monomers. The instructor also clarifies the difference between electron-donating and electron-withdrawing groups, noting their impact on the reactivity of the carbon atom in the polymerization process.
🔚 Wrapping Up Chemistry Topics for the Course
In the final paragraph, the instructor prepares to conclude the chemistry topics for the course, with plans to address any remaining questions about electron donating and electron withdrawing on Wednesday. The summary also mentions the instructor's intention to provide a supplemental lecture on resonance structures to clarify why positive charges end up in certain positions. The course will then move beyond chemistry, presumably to other areas of study.
Mindmap
Keywords
💡Ionic Polymerization
💡Cationic Polymerization
💡Anionic Polymerization
💡Living Polymerization
💡Electron Donating and Withdrawing Groups
💡Ring-Opening Polymerization
💡Carbo Cation
💡Dielectric Constant
💡Propagation
💡Termination
💡Resonance Structures
Highlights
Introduction to week 4 of Nanos, Denge Chem 134, wrapping up chemical structures and moving to thermodynamics.
Explaining the concept of ionic polymerization as a key method of chain growth polymerization.
Differentiating between cationic and anionic polymerization based on the charge of the active center.
The unique characteristic of anionic polymerization being 'living' due to the absence of termination by combination or disproportionation.
The role of formal charge in distinguishing between radicals and anions in polymerization.
Cationic polymerization initiates with a strong acid and involves a non-reactive conjugate base.
Secondary and tertiary carbo cations are more stable than primary ones in cationic polymerization.
Electron-rich substituents are necessary to stabilize the positive charge in cationic polymerization.
The importance of the counter ion size in influencing the propagation rate in ionic polymerization.
The effect of solvent dielectric constant on the electrostatic interaction and reaction rate in polymerization.
Demonstration of how tuning the dielectric constant of the solvent affects the reaction rate constant.
Kinetic chain length in anionic polymerization and its equivalence to the number average degree of polymerization.
The sensitivity of anionic polymerization to water and the need for a dry reaction environment.
Use of alkyl lithium reagents in anionic polymerization and safety considerations.
The controlled nature of anionic polymerization allowing for the creation of complex polymeric structures.
The impact of solvent polarity on the rate of anionic polymerization and the dissociation of counter ions.
Electron withdrawing groups' role in anionic polymerization and their effect on the growing chain end.
Ionic ring-opening polymerization techniques for synthesizing polymers like polyethylene oxide.
Clarification on electron donating and withdrawing groups in the context of polymerization.
The practical applications and theoretical contributions of the discussed polymerization methods.
Transcripts
Browse More Related Video
Ep10 Alkenes and pi bonds, block copolymers, dendrimers - UC San Diego - NANO 134 Darren Lipomi

Polymers - Basic Introduction

Polymer | classification of polymer on the basis of Synthesis | engineering chemistry | Mohan dangi

Ep7 free radical and controlled radical polymerization UCSD NANO 134 Darren Lipomi

Lec-08 I Types of organic reactions I Applied Chemistry I Chemical engineering

Polymer | Cationic mechanism | Addition polymerizations | engineering chemistry | Mohan dangi
5.0 / 5 (0 votes)
Thanks for rating: