Hess's Law and Heats of Formation
TLDRIn this educational video, Professor Dave introduces Hess's Law, a fundamental principle in thermochemistry that allows for the manipulation of thermochemical equations to predict energy changes in chemical reactions. He explains two methods to calculate ΔH for any reaction: by manipulating known equations and by using standard enthalpies of formation. The video illustrates how to adjust coefficients and reverse reactions to derive the desired ΔH, as well as how to calculate it using standard heats of formation found in textbooks or online. The tutorial aims to prevent unexpected chemical reactions like explosions and encourages viewers to subscribe for more educational content.
Takeaways
- 🔬 Hess's Law allows for the manipulation of thermochemical equations to predict energy changes in chemical reactions.
- ⚠️ Understanding energy changes is crucial to prevent unexpected explosions in chemical processes.
- 🔄 If the direction of a reaction is reversed, the sign of its ΔH (enthalpy change) is also reversed.
- 📈 Multiplying the molar quantities in a thermochemical equation by a coefficient also multiplies ΔH by that coefficient.
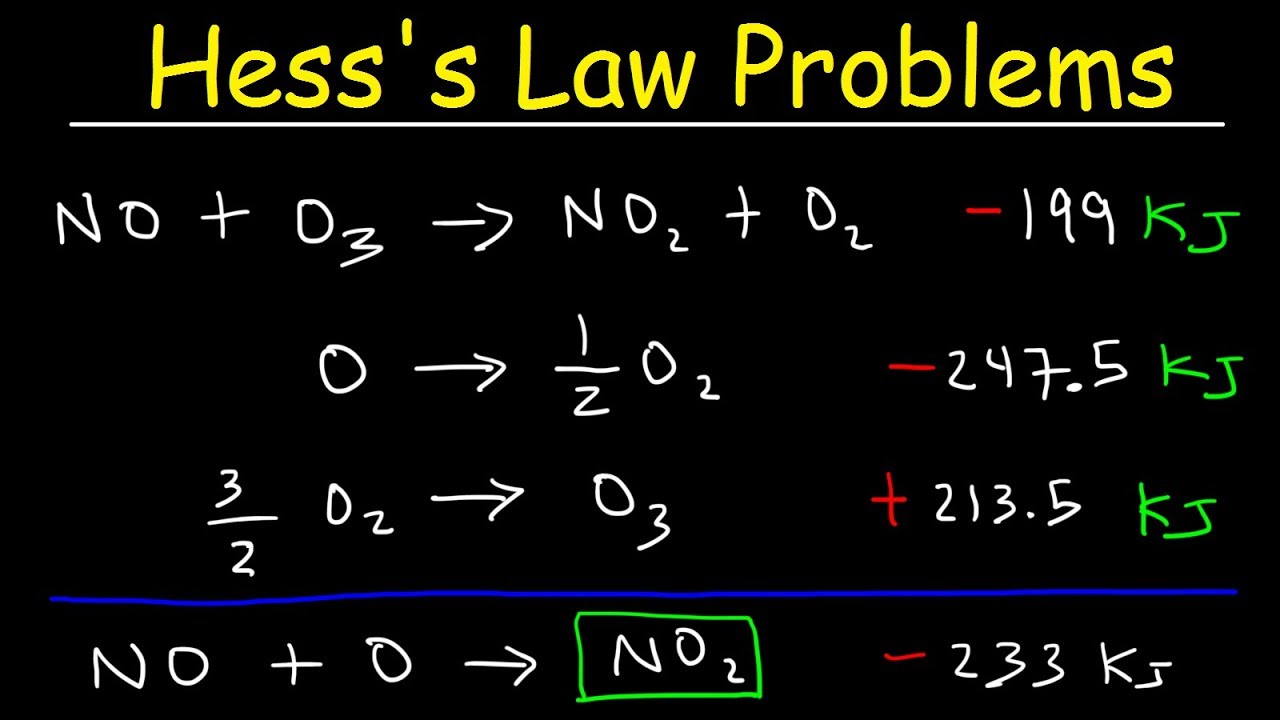
- 🧩 By rearranging and adding equations with known ΔH, we can calculate the ΔH of a reaction that is difficult to measure experimentally.
- 📚 The first provided reaction example involves doubling the equation to align with the desired reaction, thus doubling ΔH.
- ➕ Adding thermochemical equations also involves adding their respective ΔH values to find the overall ΔH for the combined reaction.
- 🔢 Hess's Law permits the manipulation of reaction coefficients and direction changes, provided the associated ΔH is adjusted appropriately.
- 📘 Standard enthalpies of formation (ΔHf°) represent the enthalpy change for forming one mole of a substance from its elements in their standard state.
- 🌡️ Standard state refers to standard temperature and pressure, typically room temperature and atmospheric pressure at sea level.
- 📊 The change in enthalpy for a reaction can be calculated by summing the standard heats of formation of the products and subtracting the sum of the standard heats of formation of the reactants.
Q & A
What is Hess's Law and why is it important in studying chemical reactions?
-Hess's Law states that the total enthalpy change (ΔH) for a chemical reaction is the same, no matter how many steps or what pathway the reaction takes to reach the final products. It's important because it allows us to predict the amount of energy absorbed or released by a reaction, which is crucial for safety and controlling chemical processes.
How can thermochemical equations be manipulated to calculate ΔH for a reaction?
-Thermochemical equations can be manipulated by reversing the reaction, multiplying the reaction by a coefficient, or adding/subtracting equations to align with the desired reaction. These manipulations must be accompanied by appropriate changes to the ΔH values to maintain the accuracy of the calculations.
What is the significance of the reverse of a reaction having the opposite ΔH?
-The reverse of a reaction having the opposite ΔH is significant because it allows for the calculation of enthalpy changes for reactions that are not easily measured experimentally. It provides a way to understand the energy changes for both the forward and reverse processes.
Can you explain the concept of multiplying molar quantities in a thermochemical equation and its effect on ΔH?
-When molar quantities in a thermochemical equation are multiplied by a coefficient, the ΔH for that equation is also multiplied by the same coefficient. This is because the energy change is directly proportional to the amount of substance reacting.
How can we use known enthalpy changes of reactions to find the ΔH of a difficult-to-measure reaction?
-We can use known enthalpy changes by rearranging and combining them to match the desired reaction. By adding or subtracting these equations, we can cancel out common substances and isolate the substances in our target equation, thus calculating the ΔH for that reaction.
What is the role of standard enthalpies of formation in calculating ΔH for a reaction?
-Standard enthalpies of formation are used to calculate the change in enthalpy for a reaction by summing the standard heats of formation of the products and subtracting the sum of the standard heats of formation of the reactants. This method provides a way to calculate ΔH when direct measurement is not feasible.
What does the 'most stable state' of an element refer to in the context of standard enthalpies of formation?
-The 'most stable state' refers to the most common allotrope or physical form of an element under standard conditions (room temperature and atmospheric pressure). For example, graphite is the most stable form of carbon, and diatomic oxygen is the most stable form of oxygen.
How do we find the standard heats of formation needed for calculating ΔH using standard enthalpies of formation?
-Standard heats of formation can be found in textbooks or online databases. These values are then used in calculations by multiplying each by the coefficients in the balanced chemical equation.
Can Hess's Law be applied to reactions involving solutions or gases?
-Yes, Hess's Law can be applied to reactions involving solutions, gases, solids, and liquids. The law is general and applies to all states of matter as long as the reaction conditions are consistent.
What is the practical application of calculating ΔH for chemical reactions in industrial processes?
-Calculating ΔH is crucial in industrial processes to ensure safety by preventing unexpected explosions or energy releases. It also helps in optimizing reactions for efficiency, minimizing energy consumption, and controlling reaction conditions.
How can the manipulation of thermochemical equations and the use of standard enthalpies of formation be combined?
-Both methods can be combined to calculate ΔH for complex reactions. Known enthalpy changes can be manipulated to form a pathway to the desired reaction, and standard enthalpies of formation can be used to fill in any gaps or to verify the calculated ΔH.
Outlines
🔬 Understanding Hess's Law and Thermochemical Equations
Professor Dave introduces Hess's Law, emphasizing its importance in predicting energy changes in chemical reactions to prevent unexpected explosions. He explains that thermochemical equations can be manipulated to calculate the enthalpy change (ΔH) of any reaction. The professor outlines two primary methods: manipulating known equations and using standard enthalpies of formation. He details the rules for manipulating thermochemical equations, such as reversing the reaction to get the opposite ΔH and adjusting ΔH proportionally with coefficients. An example is given to illustrate how to combine and adjust equations to find the ΔH of a desired reaction. The concept of standard enthalpies of formation is introduced as a method to calculate unknown ΔH by summing the products' and subtracting the reactants' standard heats of formation, which can be found in textbooks or online resources.
Mindmap
Keywords
💡Hess's Law
💡Thermochemical Equations
💡Enthalpy Change (ΔH)
💡Molar Quantities
💡Standard Enthalpies of Formation
💡Standard State
💡Coefficients
💡Rearranging Equations
💡Experimental Measurement
💡Allotropes
Highlights
Hess's Law allows manipulation of thermochemical equations to predict energy changes in chemical reactions.
Unexpected explosions can be avoided by understanding the energy changes in reactions.
There are two methods to calculate the ΔH of a reaction using tabulated thermochemical data.
The reverse of a reaction has the opposite ΔH.
Multiplying molar quantities in an equation also multiplies ΔH by the same coefficient.
Known enthalpy changes can be rearranged to find unknown changes for complex reactions.
Equations can be doubled or combined to match the desired reaction equation.
Hess's Law enables the manipulation of reaction coefficients and direction to achieve the desired ΔH.
Standard enthalpies of formation can be used to calculate unknown ΔH.
Standard enthalpies of formation represent the enthalpy of forming a substance from its elements.
Most stable state refers to the most common allotrope of an element.
Standard state is defined as room temperature and atmospheric pressure at sea level.
ΔH for a reaction can be calculated by summing standard heats of formation of products and subtracting reactants'.
Standard heats of formation can be found in textbooks or online resources.
Comprehension checks are important for understanding complex concepts like Hess's Law.
Encouragement to subscribe for more tutorials and to reach out for further questions.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: