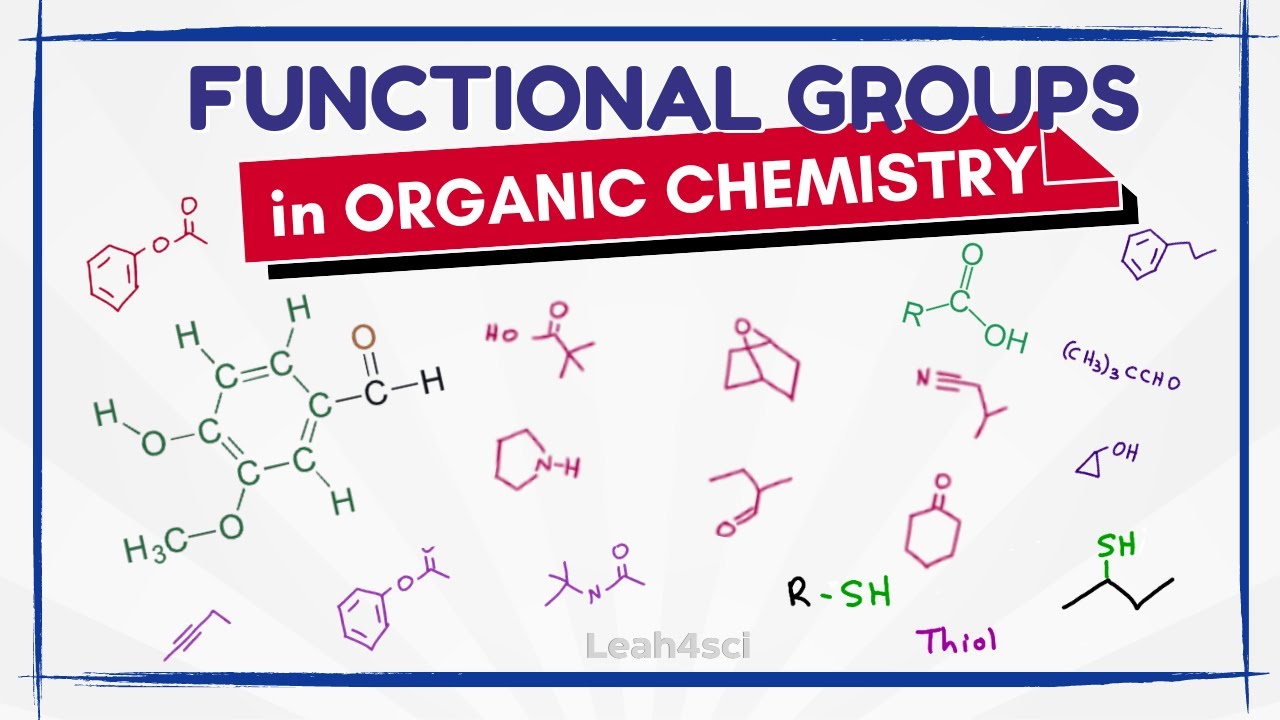
Learn Functional Groups FAST (Organic Chemistry)
TLDRThe video script offers a concise review of functional groups in organic chemistry, essential for understanding the structure and properties of organic compounds. It categorizes alkanes, alkenes, and alkynes based on the type of carbon-carbon bonds they contain, such as single, double, and triple bonds, respectively. The script then distinguishes between different functional groups including alcohols, alkyl halides, amines, and nitriles, highlighting their unique bonding patterns with carbon and other elements. It further explains the structural similarities and differences among aldehydes, ketones, carboxylic acids, esters, and amides, emphasizing the role of the attached groups in defining their chemical behavior. This overview is designed to be helpful for students, particularly those with a biology background, by drawing parallels with amino acids and simplifying complex concepts.
Takeaways
- 🧪 Alkanes are saturated hydrocarbons with single bonds between carbon atoms and hydrogen atoms.
- 🔗 Alkenes are hydrocarbons that contain a carbon-carbon double bond.
- 💠 Alkynes are hydrocarbons with a carbon-carbon triple bond.
- 🍺 Alcohols have a carbon atom bonded to a hydrogen atom and an alkyl group.
- 🧊 Alkyl halides consist of a carbon atom bonded to three other groups and a halogen from the periodic table's seventh column.
- 🌱 Amines are organic compounds with a nitrogen atom bonded to two hydrogen atoms and an alkyl group, often associated with amino acids in biology.
- 🔴 Nitriles are characterized by a carbon atom triple-bonded to a nitrogen atom.
- 🔑 The basic structure of aldehydes, ketones, carboxylic acids, esters, and amides is similar, but they differ in the groups attached to the carbonyl carbon.
- 🟡 An aldehyde has a hydrogen atom bonded to the carbonyl carbon.
- ⚫ A ketone has an R group (alkyl or aryl) bonded to the carbonyl carbon instead of hydrogen.
- 🟢 A carboxylic acid features a hydroxyl group (-OH) bonded to the carbonyl carbon.
- 🔵 An ester is formed when the hydrogen of a carboxylic acid is replaced with an R group.
- 🟣 Amides have a nitrogen atom with two other groups attached, bonded to the carbonyl carbon.
Q & A
What is the basic structure of alkanes?
-Alkanes are saturated hydrocarbons with single bonds between carbon atoms. A carbon atom with four hydrogen atoms is an example of an alkane.
How do alkenes differ from alkanes in terms of bonding?
-Alkenes differ from alkanes by containing at least one carbon-carbon double bond in addition to single bonds.
What is the defining feature of alkynes?
-Alkynes are hydrocarbons that contain a carbon-carbon triple bond.
What is the general structure of an alcohol in organic chemistry?
-An alcohol is characterized by a carbon atom bonded to a hydroxyl (OH) group.
How is an alkyl halide different from an alcohol?
-An alkyl halide is a carbon atom bonded to a halogen atom from group 17 of the periodic table, whereas an alcohol is bonded to a hydroxyl group.
What is the significance of the halogen in alkyl halides?
-Halogens are elements from the seventh group of the periodic table and are used in alkyl halides to replace hydrogen atoms in hydrocarbons.
What is the basic structure of an amine?
-An amine is characterized by a nitrogen atom bonded to two hydrogen atoms and one alkyl group or another carbon atom.
How does the structure of nitriles differ from that of alkanes?
-Nitriles differ from alkanes by having a carbon atom triple-bonded to a nitrogen atom, instead of being single-bonded to other carbon or hydrogen atoms.
What is the key difference between an aldehyde and a ketone?
-An aldehyde has a carbonyl group (C=O) bonded to a hydrogen atom, whereas a ketone has the carbonyl group bonded to an alkyl group (R group) instead of hydrogen.
How does a carboxylic acid differ from an ester in its structure?
-A carboxylic acid has a carbon bonded to a hydroxyl group (OH), while an ester has the carbon bonded to an alkoxy group (OR), where R represents an alkyl group.
What is the basic structure shared by aldehydes, ketones, carboxylic acids, esters, and amides?
-These functional groups all share a carbonyl group (C=O) as their basic structure, with different groups attached to the carbonyl carbon.
What is the main difference between an ether and an ester in terms of their functional groups?
-An ether has an oxygen atom bonded to two alkyl groups (R-O-R'), while an ester has a carbonyl group bonded to an alkyl group and an alkoxy group.
Outlines
🧪 Organic Chemistry Functional Group Review
This paragraph provides a quick review of various functional groups in organic chemistry. It categorizes alkanes as saturated hydrocarbons with single bonds, alkenes as hydrocarbons with double bonds, and alkynes as hydrocarbons with triple bonds. Alcohols are characterized by a carbon bonded to a hydrogen and an alkyl group, while alkyl halides involve a carbon bonded to three other groups and a halogen. Halogens are elements from the seventh column of the periodic table. Amines are nitrogen compounds with hydrogen and carbon or hydrogen groups attached. The paragraph also distinguishes nitriles as carbons triple-bonded to nitrogen. The speaker groups these five functional groups due to their similar basic structure, highlighting the differences in the bonding of oxygen and other elements. It concludes with a brief mention of aldehydes, ketones, carboxylic acids, esters, ethers, and amides, emphasizing their structural similarities and variations.
Mindmap
Keywords
💡Alkanes
💡Alkenes
💡Alkynes
💡Alcohols
💡Alkyl Halides
💡Amines
💡Nitriles
💡Aldehydes
💡Ketones
💡Carboxylic Acids
💡Esters
💡Amides
💡Ethers
Highlights
Alkanes are hydrocarbons with single bonds and are saturated carbons.
Alkenes contain a double bond between carbons.
Alkynes are hydrocarbons with a triple bond between two carbons.
Alcohols feature a carbon bonded to a hydrogen and an alkyl group.
Alkyl halides consist of a carbon with three other groups bonded to a halogen.
Halogens are elements in column seven of the periodic table.
Amines are characterized by nitrogen with hydrogen and alkyl groups.
Amines are often associated with amino acids in biological contexts.
Nitriles are composed of a carbon triple-bonded to a nitrogen.
The basic structure of aldehydes, ketones, carboxylic acids, esters, and amides involves a carbonyl group.
Aldehydes have a hydrogen atom bonded to the carbonyl carbon.
Ketones differ from aldehydes by having an R group instead of a hydrogen atom.
Carboxylic acids have a carbon bonded to a hydroxyl group.
Esters are formed when the hydrogen of a carboxylic acid is replaced with an R group.
Amides are characterized by a carbonyl carbon bonded to a nitrogen with two other groups.
Ethers have an oxygen atom bonded to two R groups.
It's important not to confuse ethers with esters, as they have different structures.
The review provides a helpful summary of functional groups essential for understanding organic chemistry.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: