Fries Rearrangement
TLDRThe Fries rearrangement, discovered by Karl Fries in 1908, is a reaction that converts aryl esters into ortho and para products using Lewis or strong Brønsted-Lowry acids. It's kinetically favored to produce para products under mild conditions but shifts to ortho at higher temperatures due to aluminum complex formation. The reaction's mechanism involves complexation at the carbonyl oxygen, phenol oxygen attack, and acylium ion rearrangement. While historically significant and industrially applied, its harsh conditions and limited selectivity restrict broader use. However, the anionic Fries rearrangement, utilizing strong bases for ortho-directed metalation, has overcome selectivity issues and expanded the reaction's scope in organic synthesis.
Takeaways
- 🔍 The Fries rearrangement is a chemical reaction named after Karl Fries, who first reported it in 1908.
- 🌟 It involves the rearrangement of aryl esters to form ortho and para products, promoted by stoichiometric amounts of a Lewis or strong Brønsted-Lowry acid.
- 🔄 The reaction is sometimes reversible, allowing products to rearrange back to the starting ester and undergo isomerization.
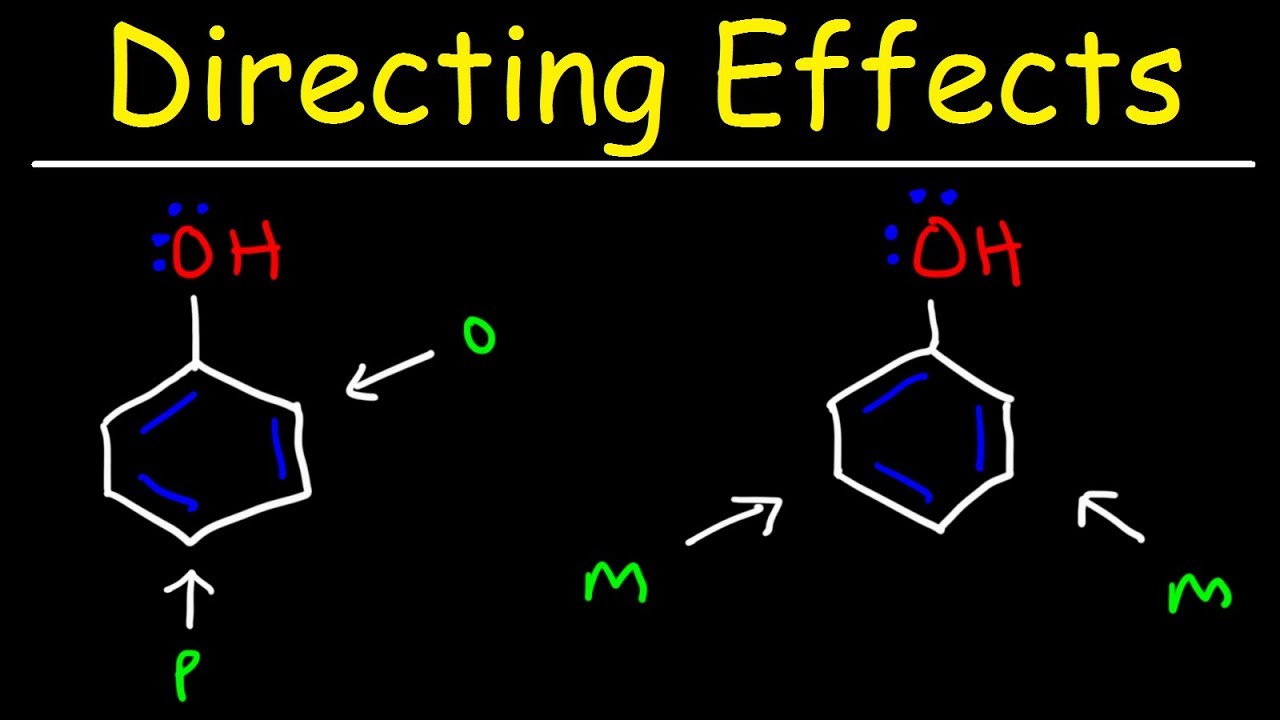
- ⏱️ The para product is kinetically favored under mild conditions, while the ortho product is thermodynamically favored at higher temperatures.
- 🔗 The mechanism begins with the complexation of the carbonyl oxygen by the acid, polarizing the bond for rearrangement.
- 💡 The acylium ion undergoes a Friedel-Crafts acylation-like reaction, influenced by the directing effect of the phenoxide group.
- 🌐 The reaction can be both intramolecular and intermolecular, as shown by crossover studies with differently labeled substrates.
- 🏭 Despite its historical importance, the Fries rearrangement is limited in application due to harsh conditions and lack of selectivity.
- 🛠️ An anionic Fries rearrangement variant using strong bases like alkyllithium in ether solvents achieves high ortho-selectivity.
- 🔑 Ortho-directed metalation is a key step in the anionic Fries rearrangement, facilitated by the initial coordination of the base with the ester oxygen.
- 🌡️ The reaction is driven by thermodynamic favorability, with the formation of a less basic phenoxide upon acyl group transfer.
- 📚 Understanding the Fries rearrangement is crucial for grasping contemporary organic synthesis and can aid in passing organic chemistry exams.
Q & A
Who discovered the Fries rearrangement reaction?
-The Fries rearrangement is named after its discoverer, German chemist Karl Fries, who first reported this reaction in 1908.
What type of compounds are involved in the Fries rearrangement?
-The Fries rearrangement involves the rearrangement of aryl esters to form both ortho and para products.
What are the conditions that promote the Fries rearrangement reaction?
-The reaction is promoted by stoichiometric amounts of a Lewis acid like AlCl3 or BCl3, or a strong Brønsted-Lowry acid like triflic acid.
Is the Fries rearrangement reaction reversible?
-Yes, the reaction is in some cases reversible, allowing the products to rearrange to give the starting ester and the possibility of isomerization between the two products.
Why is the para product kinetically favored in the Fries rearrangement?
-The para product is favored kinetically under milder conditions when the reverse reaction is slow, likely due to the directing effect of the phenoxide group.
What is the primary site of complexation for the Lewis acid in the Fries rearrangement?
-The primary site of complexation for the Lewis acid must be the carbonyl oxygen, as it is the more electron-rich and basic one.
Why is the ortho product favored at higher temperatures in the Fries rearrangement?
-At higher temperatures, the ortho product is favored, probably because the primary product before hydrolysis is an aluminum complex where the aluminum chelates the two oxygen atoms.
Why is the Fries rearrangement not catalytic?
-The reaction is not catalytic because the Lewis acid binds the product more tightly than it does the starting material.
How do crossover studies contribute to understanding the Fries rearrangement?
-Crossover studies, which involve using substrates labeled at different sites, help to determine if the reaction can be both intramolecular and intermolecular by observing the formation of crossover products.
What is the anionic Fries rearrangement and how does it differ from the traditional Fries rearrangement?
-The anionic Fries rearrangement is a variant that uses very strong bases like alkyllithium in ether solvents to metalate phenol esters at the ortho position, leading to high ortho-selectivity and a different reaction mechanism.
What is the significance of the Fries rearrangement in contemporary organic synthesis?
-The basic framework of the Fries rearrangement continues to provide new impulses to contemporary organic synthesis and is important for understanding current literature and potentially passing organic chemistry exams.
Outlines
🔬 The Fries Rearrangement: Mechanism and Historical Significance
The Fries rearrangement, discovered by Karl Fries in 1908, is a chemical reaction that transforms aryl esters into ortho and para products with the aid of Lewis or strong Brønsted-Lowry acids. The reaction is reversible and can lead to isomerization between products. Kinetically, the para product is favored under mild conditions, while the ortho product is thermodynamically preferred at higher temperatures due to the chelation of the aluminum complex with the two oxygen atoms. The mechanism involves the complexation of the carbonyl oxygen by the acid, polarization of the bond, and the formation of an acylium ion that undergoes a Friedel-Crafts acylation-like reaction. The reaction's ortho-selectivity can be improved through anionic Fries rearrangement using strong bases like alkyllithium in ether solvents, which leads to 'ortho-directed metalation' of aromatics. This method has been adapted for various substrates and heteroatoms, contributing to contemporary organic synthesis and remaining relevant for educational purposes.
📚 Applications and Limitations of the Anionic Fries Rearrangement
While the traditional Fries rearrangement has limitations due to its harsh conditions and lack of selectivity, the anionic variant offers a solution for achieving high ortho-selectivity. This method involves the use of carbamates instead of phenol esters to avoid unwanted reactions and yields ortho-hydroxy carboxamides upon reaction. The concept of ortho transfer has been successfully expanded to various groups beyond carbonyls, and the reaction is applicable to aniline systems as well. Despite the challenges with phenol esters, the anionic Fries rearrangement has been extensively reviewed and applied in organic synthesis, making it an important topic for understanding current literature and potentially acing organic chemistry exams.
Mindmap
Keywords
💡Fries rearrangement
💡Lewis acid
💡Brønsted-Lowry acid
💡Reversible reaction
💡Kinetically favored
💡Thermodynamic product
💡Chelating
💡Acylium ion
💡Crossover studies
💡Anionic Fries rearrangement
💡Ortho-directed metalation
💡Carbamates
Highlights
The Fries rearrangement is named after its discoverer, Karl Fries, who first reported the reaction in 1908.
The reaction involves the rearrangement of aryl esters to form ortho and para products.
Lewis acids like AlCl3 or BCl3, or strong Brønsted-Lowry acids like triflic acid, promote the reaction.
The reaction is sometimes reversible, allowing products to rearrange back to the starting ester.
The para product is kinetically favored under mild conditions.
At higher temperatures, the ortho product is usually favored due to the aluminum complex formation.
The reaction is not catalytic due to the Lewis acid binding the product more tightly than the starting material.
The primary site of complexation for the acid is the carbonyl oxygen due to its electron-rich nature.
The phenol oxygen attacks aluminum, initiating the acylium ion formation.
The acylium ion undergoes a Friedel-Crafts acylation-like reaction.
The reaction usually affords ortho and para structures due to the phenoxide group's directing effect.
The one equivalent of acid protonates oxygen, releasing aluminum trichloride and leaving the product.
The reaction may be partially intramolecular and partially intermolecular.
Crossover studies with labeled substrates confirm the intermolecular aspect of the reaction.
The Fries rearrangement has historical importance and industrial applications.
The anionic Fries rearrangement achieves 100% ortho-selectivity using strong bases like alkyllithium.
Ortho-directed metalation is a key process in the anionic Fries rearrangement.
Carbamates are more productive in the anionic Fries rearrangement than phenol esters.
The concept of ortho transfer has been extended to various groups and heteroatoms.
The Fries rearrangement is fundamental for understanding contemporary organic synthesis.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: