Eutectic, hypoeutectic and hypereutectic alloys
TLDRThe video discusses phase diagrams with a focus on eutectic systems, specifically the lead-tin system. It explains key concepts like eutectic temperature (183°C), eutectic composition (62% tin), eutectic alloys, and the differences between hypoeutectic and hypereutectic alloys. The microstructural evolution of these alloys during cooling is detailed, highlighting the formation of alpha and beta phases, proeutectic alpha, and eutectic mixtures. Lever rule calculations are used to determine the proportions of these phases and microconstituents in the alloy, providing a comprehensive understanding of their behavior in phase diagrams.
Takeaways
- 📊 The script discusses the eutectic phase diagram, specifically focusing on the lead-tin system as an example of a eutectic system.
- 🌡️ The eutectic temperature (T_e) for the lead-tin system is identified as 183 degrees Celsius, where the eutectic reaction occurs.
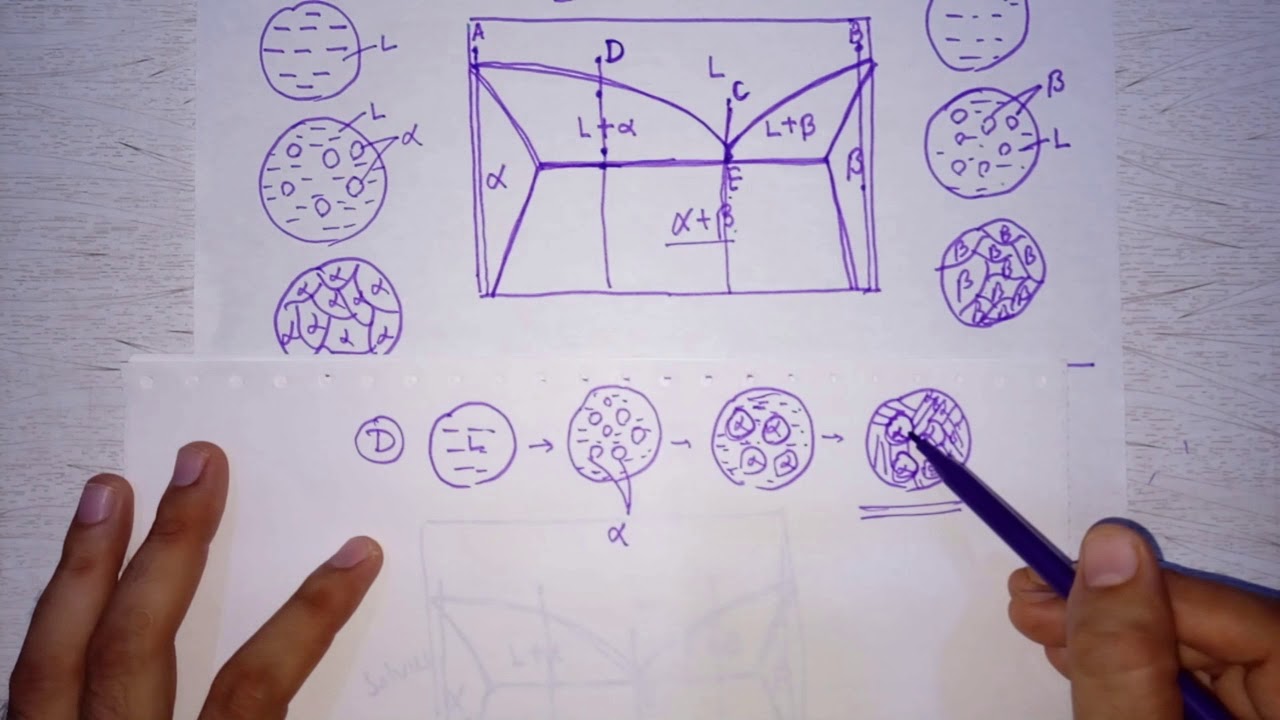
- 📍 The eutectic point in the phase diagram is a critical point, representing the lowest temperature on the liquidus line and the composition at this point is the eutectic composition (C_e).
- 🔍 The eutectic composition for the lead-tin system is 62 weight percent tin, which forms the eutectic alloy with unique properties.
- 🍽️ The eutectic alloy solidifies into a microstructure known as a eutectic mixture, which is a mixture of two phases, alpha and beta, in alternating plates.
- 📉 Hypoeutectic and hypereutectic alloys are defined in relation to the eutectic composition, with hypoeutectic having less than 62% tin and hypereutectic having more.
- 🧬 The microstructure evolution for hypoeutectic alloys involves initial formation of alpha phase as the alloy cools, followed by the formation of a eutectic mixture just below the eutectic temperature.
- 🔄 The script explains the process of determining the micro constituents of an alloy, such as proeutectic alpha and eutectic mixture, using the phase diagram and the lever rule.
- 🔢 The lever rule is applied to calculate the fractions of different phases in the alloy, such as the percentage of proeutectic alpha and eutectic mixture in a hypoeutectic alloy.
- 🧐 The distinction between phases and micro constituents is highlighted, with micro constituents being observable under a microscope, such as proeutectic alpha and eutectic mixture.
- 📝 The script provides a detailed method to calculate the total alpha and beta in an alloy, including the eutectic alpha, using the phase diagram and the lever rule.
Q & A
What is a eutectic phase diagram?
-A eutectic phase diagram is a graphical representation of the phase behavior of a binary alloy system, showing the temperatures and compositions at which different phases coexist in equilibrium.
What is the significance of the eutectic point in a phase diagram?
-The eutectic point is the minimum on the liquidus curve, representing the lowest temperature at which the liquid phase coexists with solid phases in a eutectic system. It is a critical point in the phase diagram, indicating the eutectic temperature and composition.
What are the eutectic, hypoeutectic, and hypereutectic alloys?
-Eutectic alloy is one with a composition exactly at the eutectic point. Hypoeutectic alloy has a composition less than the eutectic composition, meaning it contains less of the second element compared to the eutectic mixture. Hypereutectic alloy has a composition greater than the eutectic composition, containing more of the second element.
What is the eutectic temperature for the lead-tin system discussed in the script?
-The eutectic temperature for the lead-tin system is 183 degrees Celsius.
What is the composition of the eutectic alloy in the lead-tin system?
-The eutectic alloy in the lead-tin system has a composition of 62 weight percent tin.
How does the microstructure of a eutectic alloy evolve during solidification?
-A eutectic alloy remains liquid up to the eutectic temperature, and upon cooling, solidification begins, forming a mixture of two phases, alpha and beta, which typically appear as alternating plates in the microstructure.
What is the term used to describe the microstructure of a eutectic alloy?
-The microstructure of a eutectic alloy is referred to as a eutectic mixture, which is a mixture of alpha and beta phases, not a single phase.
How does the microstructure of a hypoeutectic alloy differ from that of a eutectic alloy?
-In a hypoeutectic alloy, solidification begins with the formation of alpha phase at temperatures above the eutectic temperature. As the alloy cools further, the remaining liquid transforms into a mixture of alpha and beta phases, forming a eutectic mixture just below the eutectic temperature.
What is the term used for the alpha phase that forms before the eutectic reaction in a hypoeutectic alloy?
-The alpha phase that forms before the eutectic reaction in a hypoeutectic alloy is called proeutectic alpha.
How can you determine the amount of proeutectic alpha and eutectic mixture in a hypoeutectic alloy?
-To determine the amount of proeutectic alpha and eutectic mixture, you draw a tie line just above the eutectic temperature in the phase diagram and apply the lever rule to find the fraction of each phase.
How can you calculate the total amount of alpha and beta phases in a hypoeutectic alloy?
-To calculate the total amount of alpha and beta phases, draw a tie line just below the eutectic temperature in the two-phase field of alpha plus beta, and then apply the lever rule to find the percentage of each phase in the alloy.
What is the difference between proeutectic alpha and eutectic alpha in the context of a hypoeutectic alloy?
-Proeutectic alpha refers to the alpha phase that forms just above the eutectic temperature, while eutectic alpha is the alpha phase that is part of the eutectic mixture, which forms just below the eutectic temperature.
Outlines
📚 Eutectic Phase Diagram and Alloys
The paragraph introduces the concept of a phase diagram, with a focus on the eutectic phase diagram. It explains the lead-tin system as an example of a eutectic system, highlighting the eutectic composition and the eutectic alloy. The hypoeutectic and hypereutectic alloys are also mentioned, emphasizing the importance of the eutectic point and temperature. The paragraph describes the microstructure evolution of eutectic alloys, which solidify into a mixture of two phases, alpha and beta, forming an alternating plate structure known as a eutectic mixture.
🔍 Microstructure Evolution in Hypoeutectic Alloys
This section delves into the microstructure evolution of hypoeutectic alloys, using a 40% tin alloy as an example. It explains how such an alloy, with a composition less than the eutectic point, begins to form alpha phase upon cooling and continues to do so until the eutectic temperature is reached. The paragraph discusses the process of alpha formation and the eventual transformation of the remaining liquid into a mixture of alpha and beta phases just below the eutectic temperature, leading to a microstructure consisting of proeutectic alpha and eutectic mixture.
🌡️ Liquid Composition and Phase Transformation
The paragraph discusses the evolution of the liquid composition in a hypoeutectic alloy as it cools. It explains how the initial liquid composition changes as the alloy cools and reaches the liquidus, following the liquidus line until it reaches the eutectic composition just above the eutectic temperature. The paragraph also describes the eutectic reaction, where the liquid of eutectic composition transforms into a mixture of alpha and beta phases, resulting in a microstructure with proeutectic alpha and eutectic mixture as the two micro constituents.
🔬 Determining Micro Constituents in Hypoeutectic Alloys
This paragraph explains how to determine the micro constituents of a given hypoeutectic alloy, specifically focusing on the amount of proeutectic alpha and eutectic mixture. It describes the process of drawing a tie line just above the eutectic temperature to calculate the fraction of proeutectic alpha formed before the eutectic reaction. The summary also includes the calculation of the eutectic mixture fraction and clarifies the difference between the phases and micro constituents in the context of microstructure and phase diagrams.
📏 Lever Rule Application for Total Alpha and Beta
The paragraph describes the application of the lever rule to determine the total amounts of alpha and beta in a hypoeutectic alloy. It explains the process of drawing a tie line just below the eutectic temperature to calculate the total alpha and beta phases present in the alloy. The summary includes the calculation of the percentage of the alloy that is in the form of alpha and beta, providing a clear understanding of the distribution of phases in the alloy.
📉 Calculating Eutectic Alpha in the Alloy
This final paragraph focuses on calculating the amount of alpha that is part of the eutectic mixture. It explains how to find the eutectic alpha by subtracting the proeutectic alpha from the total alpha. The summary provides the calculation and the resulting percentage of alpha that is part of the eutectic mixture, completing the analysis of the alloy's microstructure.
Mindmap
Keywords
💡Eutectic Phase Diagram
💡Eutectic Point
💡Eutectic Alloy
💡Hypoeutectic Alloy
💡Hypereutectic Alloy
💡Microstructure
💡Proeutectic Alpha
💡Eutectic Mixture
💡Lever Rule
💡Liquidus Line
Highlights
Discussion of the eutectic phase diagram, specifically the lead-tin system.
Introduction of eutectic composition and eutectic alloy, and related terms hypoeutectic and hypereutectic alloys.
Explanation of the significance of the eutectic point and eutectic temperature in phase diagrams.
Description of the microstructure evolution for eutectic alloys and the formation of alternating plates of alpha and beta.
Differentiation between eutectic mixture, a micro constituent, and the actual phases.
Microstructure evolution for hypoeutectic alloys and the formation process of alpha phase before the eutectic reaction.
Illustration of the cooling process for a 40% tin hypoeutectic alloy and the formation of proeutectic alpha.
Discussion on the transformation of liquid to alpha and beta just below the eutectic temperature.
Analysis of the liquid composition evolution and its reaching the eutectic composition at temperatures just above T_e.
Explanation of the eutectic reaction where liquid of eutectic composition transforms into a mixture of alpha and beta.
Identification of two micro constituents in a hypoeutectic alloy: proeutectic alpha and eutectic mixture.
Clarification of the difference between phases and micro constituents in the context of microstructure and phase diagrams.
Method to determine the micro constituents of a given alloy using tie lines and the lever rule.
Calculation of the fraction of proeutectic alpha in a 40% tin alloy and its significance.
Determination of the total alpha and beta in the alloy using the lever rule just below the eutectic temperature.
Explanation of how to find the amount of alpha that is part of the eutectic mixture.
Final summary of the microstructure of a 40% tin hypoeutectic alloy, including percentages of proeutectic alpha, eutectic mixture, total alpha, and total beta.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: