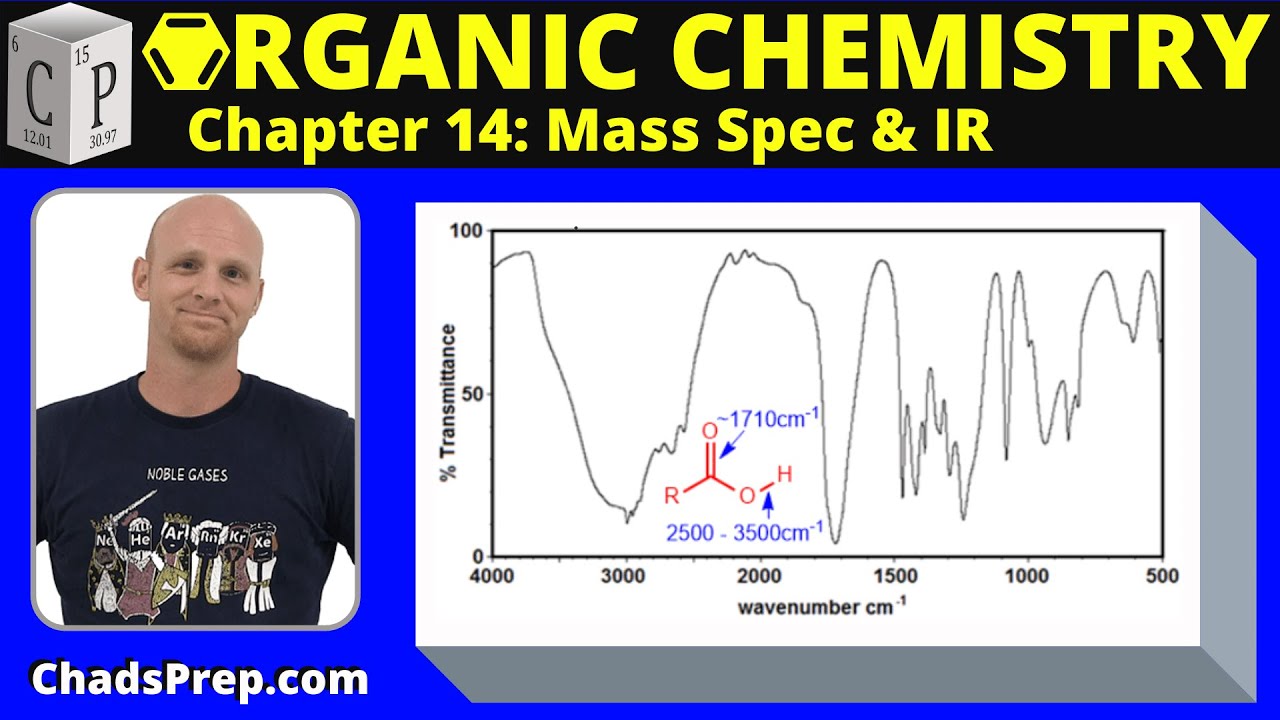
14.2b The Effect of Conjugation on the Carbonyl Stretching Frequency | Organic Chemistry
TLDRThe transcript delves into the chemistry of carbonyl compounds, specifically focusing on the impact of conjugation on the stretching frequencies of carbon-oxygen double bonds. It explains that conjugation, which involves a pattern of double and single bonds, leads to electron delocalization and a decrease in stretching frequencies for carbonyl groups. The discussion covers various resonance structures, illustrating how minor contributors can affect the stability and bond strength of these compounds. Amides are highlighted for their unique behavior due to the lone pair on nitrogen, which results in lower stretching frequencies compared to esters. The summary emphasizes the importance of understanding the nuances of conjugation and resonance, rather than memorizing general rules, to accurately predict the behavior of different chemical bonds.
Takeaways
- 📊 Conjugation, which involves a double bond followed by a single bond and another double bond, can lower the stretching frequencies of carbonyl groups due to electron delocalization.
- 🔬 The presence of a benzene ring in conjugation with a carbonyl group can further decrease the stretching frequency to around 1691 cm⁻¹.
- ⚖️ Resonance structures with charge separation are less stable than those without, impacting the overall stability and frequency of the carbonyl bond.
- 🔍 Minor resonance contributors can explain the differences in stretching frequencies observed in various carbonyl-containing compounds.
- 🔬 In the case of amides, the lone pair on nitrogen allows for an additional resonance structure, which can lower the stretching frequency even further compared to esters.
- 📉 The stretching frequency of a carbonyl bond is influenced by the degree of double bond character; a stronger double bond character results in a higher frequency.
- 🔬 Ester carbonyl bonds, despite having a resonance structure similar to amides, shift to a higher frequency due to the electronegativity of oxygen destabilizing the resonance structures.
- 📚 Memorizing that resonance always lowers stretching frequencies is not accurate; this is generally true for carbonyls but may not hold for other types of bonds.
- 🔬 The major resonance contributor for a polar carbon-oxygen bond typically has no charge separation and is more stable than minor contributors.
- 📐 The percentage representation of double bond character in the resonance hybrid can vary, affecting the bond's strength and stretching frequency.
- 📉 A higher percentage of double bond character in a carbonyl bond results in a stronger bond and a higher stretching frequency, as seen in the comparison between different compounds.
Q & A
What is conjugation in the context of chemical compounds?
-Conjugation refers to the arrangement of a double bond followed by a single bond and another double bond within a molecule, allowing for the delocalization of electrons across these bonds.
How does conjugation affect the stretching frequencies of carbonyl groups?
-Conjugation can lower the stretching frequencies of carbonyl groups due to the delocalization of electrons, which results in a decrease in the bond's double bond character.
What is the significance of resonance structures in understanding bond characteristics?
-Resonance structures help explain the distribution of electrons and the stability of molecules. They can indicate the degree of double bond character in a molecule, which in turn affects its reactivity and bond strength.
How does the presence of a benzene ring affect the stretching frequency of a carbonyl group?
-When a carbonyl group is conjugated with a benzene ring, the stretching frequency decreases because of the additional resonance structures that result from the interaction between the pi electrons of the benzene ring and the carbonyl group.
Why do amides have a lower stretching frequency compared to other carbonyl compounds?
-Amides have a lower stretching frequency because the lone pair on the nitrogen atom can participate in resonance, creating additional resonance structures that decrease the double bond character of the carbonyl group.
What is the impact of electronegativity on the stability of resonance structures?
-Electronegativity can influence the stability of resonance structures. For instance, the more electronegative oxygen in an ester destabilizes the resonance structures compared to amides, leading to a higher stretching frequency.
How does the presence of a carbon-carbon double bond affect the stretching frequency of an adjacent carbonyl group?
-The presence of a carbon-carbon double bond adjacent to a carbonyl group can lead to conjugation, which in turn lowers the stretching frequency of the carbonyl group due to the delocalization of electrons.
What is the role of minor resonance contributors in the overall structure of a molecule?
-Minor resonance contributors, although less stable than the major resonance structure, still play a role in the overall hybrid structure of a molecule. They can slightly alter the bond characteristics, such as bond strength and stretching frequency.
Why might a professor ask about conjugation in relation to bonds other than carbonyls?
-A professor might ask about conjugation in relation to other bonds to ensure students understand that the effect of conjugation on bond characteristics can vary depending on the type of bond and the specific molecular context.
What is the general rule regarding the stretching frequencies of carbonyl bonds and conjugation?
-The general rule is that conjugation with a carbonyl bond typically lowers the stretching frequency due to the increased delocalization of electrons and the decrease in double bond character.
How can one determine the major and minor resonance contributors in a molecule?
-One can determine the major and minor resonance contributors by analyzing the distribution of electrons and the stability of different possible structures. The most stable structure with the least charge separation is usually the major contributor.
Why is it important to not just memorize rules about bond characteristics without understanding the underlying concepts?
-It's important to understand the underlying concepts because exceptions to general rules can occur. Understanding allows for the ability to predict and explain behavior in different molecular contexts, rather than relying on memorized but potentially inaccurate rules.
Outlines
📚 Conjugation and Carbonyl Bond Frequencies
The first paragraph discusses the impact of conjugation on the stretching frequencies of carbonyl bonds. It explains that conjugation, which involves a double bond, single bond, and another double bond pattern, leads to electron delocalization and lowers the stretching frequencies for carbonyl groups. The paragraph uses examples of butanone and benzene to illustrate how the presence of an alkene or a benzene ring can affect these frequencies. It also touches on the resonance structures of amides and how the lone pair on nitrogen contributes to their low shift frequencies. The explanation includes a hypothetical percentage contribution of different resonance structures to the overall stability and bond character, emphasizing that memorizing general rules without understanding the underlying concepts is not advisable.
🔬 Ester and Amide Bond Resonance Comparison
The second paragraph compares the resonance structures and stretching frequencies of esters and amides. It highlights that the electronegativity of oxygen in esters destabilizes the resonance structures compared to amides, where nitrogen is less electronegative. This difference results in esters having less stable resonance contributors and thus a higher stretching frequency. The paragraph also discusses the impact of the lone pair on nitrogen in amides, which allows for additional resonance structures that affect the bond character and stretching frequency. The summary concludes by noting that esters, despite having less stable resonance contributors, end up with a higher frequency due to the destabilizing effect of the electronegative oxygen.
Mindmap
Keywords
💡Conjugation
💡Delocalization
💡Resonance
💡Stretching Frequencies
💡Carbonyl Bond
💡Resonance Hybrid
💡Electronegativity
💡Amide
💡Ester
💡Ketone
💡Benzene Ring
Highlights
Conjugation occurs when there is a double bond, single bond, double bond pattern, leading to delocalization of electrons and lower stretching frequencies for carbonyls.
Butanone with a carbonyl conjugated with an alkene shows a signal at 16.95, lower than the non-conjugated carbonyl signal at 17.18.
Conjugation with a benzene ring further lowers the carbonyl stretching frequency to 16.91.
Amides show even lower carbonyl stretching frequencies around 16.74-16.85 due to conjugation with the nitrogen lone pair.
Resonance structures help explain the differences in stretching frequencies for various carbonyl compounds.
Minor resonance contributors can significantly impact the carbonyl stretching frequency.
The major resonance contributor with no charge separation is more stable than structures with charge separation.
The degree of double bond character in the resonance hybrid influences the carbonyl stretching frequency.
A stronger carbon-oxygen double bond results in a higher stretching frequency, while a weaker bond leads to a lower frequency.
Memorizing that conjugation always lowers stretching frequencies is not accurate for all bonds, but it generally holds true for carbonyls.
For carbonyls, increased conjugation strength leads to lower stretching frequencies, while decreased double bond character results in higher frequencies.
Conjugation with a benzene ring in acetophenone also lowers the carbonyl stretching frequency.
In amides, the lone pair on nitrogen allows for additional resonance structures, further lowering the carbonyl stretching frequency.
Electronegativity differences between nitrogen and oxygen impact the stability of resonance structures and stretching frequencies.
Esters have a higher carbonyl stretching frequency at 1735 cm-1 due to less stable resonance contributors compared to amides.
Understanding the impact of conjugation and resonance structures on carbonyl stretching frequencies is crucial for interpreting IR spectra.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: